AP State Syllabus AP Board 7th Class Science Important Questions Chapter 17 Changes Around Us
AP State Syllabus 7th Class Science Important Questions 17th Lesson Changes Around Us
7th Class Science 17th Lesson Changes Around Us Important Questions and Answers
Question 1.
What are periodic changes?
Answer:
| S.No. | Name of the Change |
| 1. | Change of day and night |
| 2. | Withering of leaves |
| 3. | Rising of the pole star |
| 4. | Change of Seasons |
| 5. | Change of Greenery in the fields of cultivation |
| 6. | Changes in lengths of shadows |
| 7. | Appearance of Full Moon |
- If we observe that every change mentioned is the table repeats after some period of time.
- Such changes are known as periodic changes.
- The events which repeat at regular intervals of time are called periodic events.
![]()
Question 2.
Mention some physical changes you observe in your daily life.
Answer:
- In our daily life we observe many changes.
- In the changes like melting of ice, solidification of ghee or coconut oil in winter etc., there is a change in state of the substance.
- In certain processes like filling balloons with air and pumping of cycle tubes etc., we notice change in shape.
- These are all physical changes. On these changes no new substance is formed.
Question 3.
Explain what are physical and chemical changes. Give examples.
Answer:
Physical change: When the material undergoes a change in shape, size, colour or state, it is called a physical change.
Eg: Heating ice, filling air into Balloons
Chemical change: When a material undergoes a change in shape and size and a new material is formed, then we call it a chemical change.
Eg:
- Burning of crackers
- Change of milk into curd
- Burning a piece of wood.
Question 4.
Some changes are given in the table. Write possible changes you notice for each case and put (✓) in the appropriate column.
Answer:
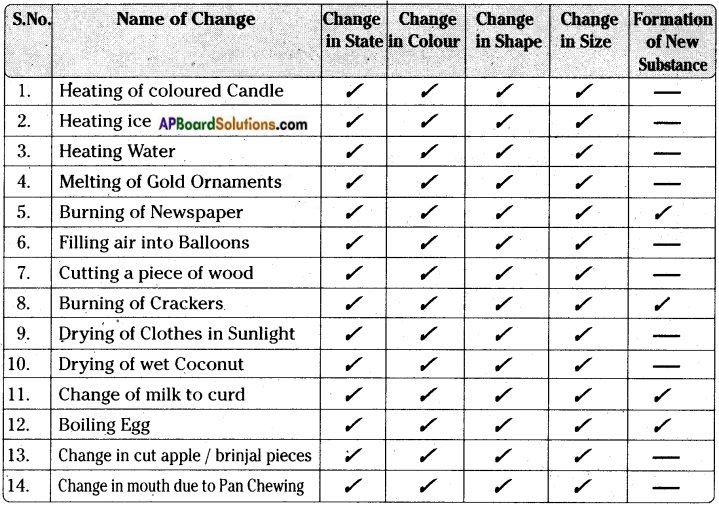
- In the above activity we notice that only in some examples like burning of paper, burning of crackers, change of milk to curd, boiling of egg, etc., a new substance is formed.
- But in other examples of changes, we notice a change in state or colour or size or shape etc but the substance remains the same and no new substance is formed.
![]()
Question 5.
In the above table which changes do you consider physical changes or chemical changes?
Answer:
- When a material undergoes a change in shape, size, colour, or state, it is called a physical change.
- So I consider the following as physical changes.
Heating a coloured candle, Heating ice, Heating water, Melting of Gold ornaments, Filling of air into Balloons, Cutting a piece of wood, Drying of clothes in sunlight, Drying of wet coconut, change is the cut apple; Change in the mouth due to pan chewing. - When a material undergoes a change in its composition, it is called a chemical
change. In a chemical change, a new substance is formed. - So I consider the following as chemical changes. Burning of Newspaper, Burning of crackers, change of milk to curd, Boiling egg.
Question 6.
What is rust and rusting? Why does iron rust? What type of change is this?
Answer:
- When iron nails, iron gates, iron benches or pieces of iron are left is the open ground for a long time, we find a brown layer on the surface of iron articles.
- This is called ‘rust’, and the process of forming this layer is called rusting.
- When iron is exposed to air for a long time, the Oxygen present in air reacts with it in the presence of moist air and forms a new substance called iron oxide as rust on iron articles.
- This process is known as rusting.
Iron + Oxygen (from air) + Water → rust (Iron oxide). - As a new substance is formed in this change, we call it a chemical change.
Question 7.
What is Galvanisation? Explain its importance.
Answer:
- Some articles made up of iron, don’t get rusted even they are exposed to air.
- To prevent iron articles from coming into contact with oxygen in air or water or both, we deposit a layer of a metal like Chromium or Zinc on them.
- This process of depositing a layer of zinc or Chromium on Iron is called Galvanisation.
- Generally we use Zinc for such type of coatings.
- We find in our house that water pipe lines are without rust on them.
- If we observe carefully, we notice that there is some metallic coating on these pipes to prevent rusting.
- They do not get rusted even after a long time since they are galvanized.
- The process of depositing one metal on another metal is called galvanisation.
Question 8.
What happens when you burn camphor?
Answer:
- Initially Camphor changes into liquid and then evaporates into air.
- It is also considered to be a chemical change.
![]()
Question 9.
What happens when you put a small quantity of Camphor in a dish and place it in the open air?
Answer:
- Take a small quantity of Camphor in a dish and place it in the open air.
- Observe it after some time. Its quantity reduces and we sense the smell of it.
- It happens because the camphor gets evaporated. Since it has strong smell, it is used to keep insects and flies away. It is also used in medicines.
Question 10.
Describe what changes occur in a chemical change.
Answer:
- In a chemical change, the material undergoes a change in its composition and a new substance is formed.
- Heat, light or any other radiation may be given out or absorbed.
- Loud sound may be produced.
- A change in smell may take place or a new smell may be produced.
- A colour change may take place.
- A change in the state may occur. All chemical changes do not have all the 2 to 6 traits mentioned above.
Question 11.
How do you make crystallization of urea?
Answer:
- Take some water in a test tube and add urea to it. Heat the test tube till all the urea dissolves.
- Add more urea to it. Keep on adding to it until no more urea can be dissolved in it.
- Let the solution cool down for sometime.
- Observe the test tube after about half an hour.
- We get large size crystals of urea.
Question 12.
What is crystallization? How do you make crystallization of copper sulphate?
Answer:
Crystallization (Definition): The process of separating a soluble solid from the solution by heating or evaporating is called crystallization.
Crystallization of copper sulphate:
- Take some hot, saturated solution of Copper sulphate in a test tube.
- Pour some of it in an evaporating dish.
- Allow the solution to cool quickly.
- Observe with a magnifying glass, the size, colour and shape of the crystals formed.
Question 13.
Think about the following changes and decide whether they are physical or chemical changes. Write the type of change and reasons for that in the table.
Answer:
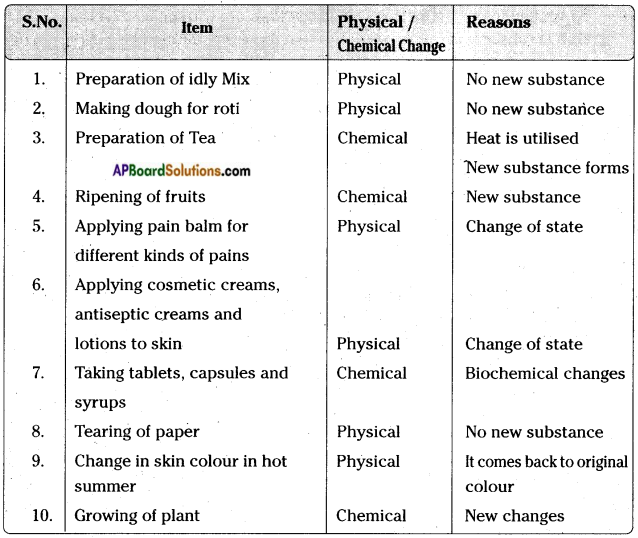
![]()
Question 14.
Do copper articles get rust? When rusting becomes faster? What change is this?
Answer:
- When Copper utensils are exposed to air we find a greenish coat on them.
- This greenish coat is formed when Copper reacts with Oxygen and Carbon dioxide present in the air.
- This coat also protects Copper from getting further corroded. It is also an example of rusting.
- Rusting becomes faster when there is more humidity in the air.
- Rusting is a chemical change.
Question 15.
How can you say that rusting is a chemical change? What factors are favourable for speed rusting?
Answer:
- In case when the metal is changed to its oxide, that is rusting a new substance is formed.
- Hence rusting is a Chemical Change.
- The speed of rusting depends on the amount of moisture available to it for a long time.
- That is more the humidity in air, the faster is the rusting of iron.
Question 16.
What are the methods you suggest to prevent the rusting of iron?
Answer:
- The problem of rusting of iron and other metal articles is common experience in almost every home.
- It spoils beautiful articles and makes them look ugly.
- The following are some of the ways to prevent the rusting of iron.
a) Do not allow the iron articles to come in direct contact with Oxygen in the air, water or both.
b) Apply a coat of paint or grease on an iron article.
Question 17.
Do all the materials get rusted or react with oxygen in the air?
Answer:
- Observe Gold and Silver, we wear them in the form of ornaments.
- Even if they get exposed to air for a long time, they do not change colour or get rusted.
- It means that they are resistant to corrosion which is the reason why we use them in making ornaments.
Question 18.
What changes do you notice when few pieces of ice in a beaker are heated? What do you mean by a physical change ?
Answer:
- Take few pieces of ice in a beaker and heat them as shown in the following figure.

- We notice that ice slowly melts and becomes water and on further heating.
- If we reduce the temperature, the water vapour changes back to water and when the temperature is further reduced it changes to ice.
- In the above activity we notice the change of the state of ice to water and to vapour but the substance, water, remains the same.
- Changes of this type where no new substance is formed are known as physical changes.
- When a material undergoes a change in shape, size, color or state it is called a Physical Change.
- Generally, no new substance is formed in a physical change.
![]()
Question 19.
What changes do you observe when some material is burning? What do you mean by a chemical change?
Answer:
- Take a piece of wood, a piece of paper and a ball of cotton. Burn them and observe the changes.

- Record your observations in the following Table.

- In the above activity we notice that when a piece of wood, paper, and cotton are burnt a new material is formed.
- This is black in colour and in powder form which is different from the original material.
- We also notice change in shape and size of new material.
- This type of change that leads to form a new substance is known as Chemical change.
Question 20.
What happens when magnesium ribbon is burnt in air? What type of substance is formed when it is dissolved in water?
Answer:
- Take a small piece of Magnesium ribbon. Burn it on a flame of candle.
- We will find brilliant white dazzling light leaving a powdery substance behind.
- When Magnesium burns in the presence of Oxygen, it forms Magnesium Oxide in the form of powder ash, which is a new substance.
- Thus there is a change in the composition.
- Magnesium + Oxygen → Magnesium Oxide.
- Collect the ash and mix it with a small quantity of water and dissolve it. Another new substance is formed.
- Magnesium Oxide + Water → Magnesium Hydroxide
- Test the dissolved mixture with blue and red litmus papers to decide whether it is a acid or a base.
- We notice that the litmus paper turns blue.
- This means that magnesium hydroxide is basic in nature.
- As all these are new substances all these changes are chemical changes.
![]()
Question 21.
What happens if you put an iron nail in copper sulphate solution? Describe how do you perform this activity.
Answer:
- Take a glass tumbler half – filled with water and add a teaspoonful of Copper sulphate to it.
- Now add a few drops of Sulphuric Acid to the Copper Sulphate solution.
- Take some sample solution of it in another beaker and keep it aside.
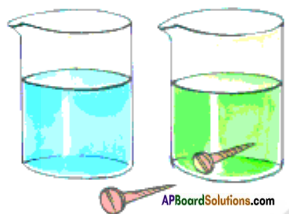
- Add an Iron nail to the solution in the first beaker and keep it undisturbed for half an hour.
- Compare the colour of the solution in which iron nail is dropped to that of sample solution kept aside.
- We notice that the blue coloured solution changes into green colour and a brown coloured deposit is seen on the iron nail.
- The change in colour of the solution is due to the formation of Iron Sulphate, a new substance.
- The brown deposit on the Iron nail is copper, another new substance.
- Copper Sulphate (Blue) + Iron → Iron Sulphate (green) + Copper (brown deposit).
- This is a chemical change.
Question 22.
Describe how do you perform the activity to observe the reaction of vinegar with baking soda.
Answer:
- First set up the apparatus has shown in Fig.
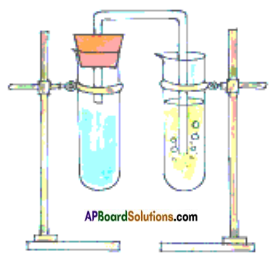
- Take a teaspoon of vinegar (acetic acid) in a test tube and add a pinch of baking soda (Sodium bicarbonate) to it.
- You observe bubbles coming out with a hissing sound. Pass this gas through freshly prepared Limewater (Calcium Hydroxide).
- Limewater changes to milky white showing that the gas sent into the test tube is Carbon dioxide.
- Vinegar + Baking Soda → Carbon dioxide + other substance.
- Carbon dioxide + Lime Water → Calcium Carbonate + Water
- In these reactions, the new substances like Carbon dioxide and Calcium Carbonate are formed. Hence it is a chemical change.
- When a material undergoes a change in its composition it is called a chemical change.
![]()
Question 23.
How do you produce large size of sugar crystals? What type of change is it?
Answer:
- Take a big size test tube. Fill half if it with water. Add some sugar to it and stir it.
- Keep adding sugar and stirring until saturation is attained. Then heat this sugar solution and add some more sugar to it while stirring
- Continue adding sugar till no more sugar can be dissolved in it.
- Now filter the solution and allow it cool for half an hour.
- We notice formation of large size crystals of sugar at the bottom of the beaker. Thus sugar the small granules of sugar added changed into large size sugar crystals.
- This is a physical change.
Question 24.
What do you observe on the cut pieces of fruits and vegetables? Why do they change their colour?
Answer:
- Take an apple, brinjal, a potato, a tomato, a cucumber, a banana; cut each into small pieces; place them in separate plates and expose them to open air for sometime.

- We notice a brown colour in some of these pieces.
- Some fruits and vegetables, when cut, react with Oxygen in the air.
- This makes them to get a brown layer on the surface.
Question 25.
What do you do to prevent browning of cut vegetables and fruits.
Answer:
- Cold water prevents the outer surface of the potato and brinjal from colouring.
- Small quantities of acids like vinegar or lemon juice in water will also prevent browning of vegetables.

- We can also rub the surface of the cut fruits with juices of citrus fruits like lemon to avoid their browning.
- The layer of lemon juice reduces the reaction on the surface of the fruit.
- Ascorbic acid (vitamin C) can also be used to prevent browning.
![]()
Question 26.
Look at the experiment given below which was already conducted in your laboratory. Answer the following.

a) Which chemical was used in this experiment?
b) What is the change of colour you observed, after putting the iron nail into the solution?
Answer:
a) Copper Sulphate solution (CuSO4).
b) The solution turns into green colour.