Students can go through AP State Board 8th Class Physical Science Notes Chapter 5 Metals and Non-Metals to understand and remember the concept easily.
AP State Board Syllabus 8th Class Physical Science Notes Chapter 5 Metals and Non-Metals
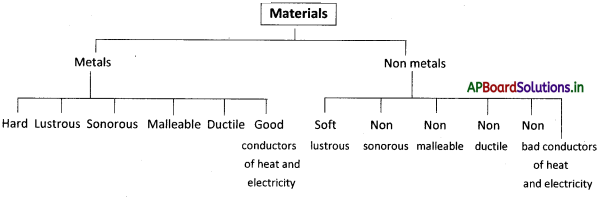
→ The material which show brightness on the surface and reflect the light are called lustrous and which do not shine are called non-lustrous material.
→ The property of materials by which they can be beaten into thin sheets is called malleability.
→ The property of drawing material to make fine wires is called ductility.
→ The ability of materials to produce a particular sound when it is dropped on the hard surface is called sonorous.
→ Metals often possess all of the following properties. They are lustrous, hard, malleable, ductile, good conductors of heat and electricity, and sonorous.
E.g.: Copper, magnesium, aluminum, iron, zinc, etc.
→ If Most of the metals exist in a solid-state except mercury which exists in a liquid state.
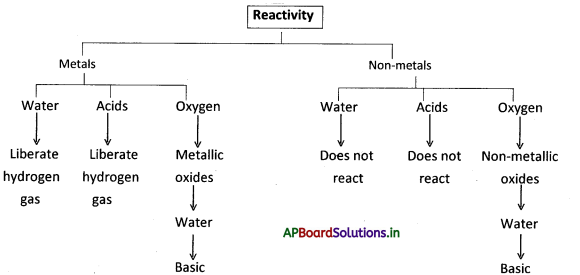
→ Some metals react with the components of air in a different manner with different rates and different conditions.
→ Gold and platinum are metals that do not react with air.
→ Metals react with acids and liberate hydrogen gas.
→ Non-metals do not react with water and acids.
![]()
→ Metals can displace each other according to their reactivity.
→ On burning metals react with oxygen to produce metal oxides which are basic in nature.
→ On burning non-metals react with oxygen to produce non-metal oxides which are acidic in nature.
→ Metals and non-metals are used widely in everyday life.
→ Metals: The material which is hard, lustrous, malleable, ductile, good conductors of heat and electricity and are sonorous are called metals.
→ Non-metals: The material which are soft, non-lustrous, non-malleable, non-ductile, bad conductors of heat and electricity, and ore non-sonorous are called non-metals.
→ Lustrous: The material which shows brightness on the surface and reflects the light is called lustrous.
→ Sonority: The property of producing particular sound materials.
→ Malleability: The property by which metals can be flattered into thin sheets is called malleability
→ Ductility: The property by which metals càn be drawn into fine fires is called ductility.
→ Good conductors of heat and electricity: The material which easily allows the passage of electricity and heat through them are called good conductors of heat and electricity.
→ Oxides of metals and non-metals: When we burn metals or non-metals in the presence of oxygen they form metallic oxides or non-metallic oxides.
![]()
→ Displacement reaction: The reaction ¡n which a more reactive element displaces a less reactive element from its compound in an aqueous solution.
→ Sonorous: The ability of materials to produce a particular sound when ¡t ¡s dropped on the hard surface is called sonorous.
→ James Smithson Tennant (1761 – 1815):
- This is the man who discovered iridium back in 1804.
- Eminent Cambridge professor in chemistry who first isolated osmium and iridium from native platinum deposits.
- He also identifIes pro of diamond and charcoaL
- The mineral tennantite was named after him.