AP State Syllabus AP Board 8th Class Physical Science Solutions Chapter 5 Metals and Non-Metals Textbook Questions and Answers.
AP State Syllabus 8th Class Physical Science Solutions 5th Lesson Metals and Non-Metals
8th Class Physical Science 5th Lesson Metals and Non-Metals Textbook Questions and Answers
Improve Your Learning
Question 1.
Explain the physical properties of metals with suitable examples.
(OR)
Explain briefly the physical properties of metals.
Answer:
Physical properties of metals:
- Lustrous:
When we rub the surface of metals with sand paper they will shine. This property is called lustrous, e.g.: Iron, zinc etc. - Sonority:
When we hit the metal surface they give a ringing sound is called sonority, e.g.: Iron, copper etc. - Malleability:
Metals can be flattened into thin sheets. The property of flattening metals into thin sheets is called malleability, e.g.: Silver, Iron, copper. - Ductility:
Metals can be drawn into wires.
The property of drawing a metal to make fine wire is called ductility, e.g.: Silver, gold. - Electric conductivity:
Electricity can be easily pass through metals. So they are called good conductors of electricity.
e.g.: Silver, copper, iron. - Conductivity of heat:
Metal absorbs heat quite easily. They are good conductors of heat, e.g.: Copper, iron.
![]()
Question 2.
You are given two samples. How do you distinguish which is metal and which is non-metal?
Answer:
- By physical properties, generally we can judge whether the given sample is metal or non-metal that is metals are hard. They are lustrous, sonorous, malleable, ductile and good conductors of heat and electricity.
- Generally metal sample react with water and non-metal sample does not react with water.
- Generally metal sample react with acid and produce hydrogen gas whereas non-metal samples does not react with acid.
Question 3.
Which metals are used in making jewellery? Why?
Answer:
Generally we use metals like gold, silver and copper for making jewellery due to their high ductility and lustrous surface.
Question 4.
Which substance liberate hydrogen when react with metals?
Answer:
Generally acids react with metals and liberate hydrogen gas. Water also react with some metals and liberate hydrogen gas.
Question 5.
In a chemical reaction iron is unable to displace zinc from zinc sulphate. Why?
Answer:
Zinc is more reactive than iron. A less reactive metal cannot displace a more reactive metal from its salt solution. So iron unable to displace zinc from zinc sulphate.
![]()
Question 6.
Why cooking pans do not have metal handles?
Answer:
Metals are good conductors of heat. So they gain heat easily. So in order to avoid the burning of our palms, the handles of cooking pans made of heat resist material such as plastic.
Question 7.
Sulphur dioxide is
A) basic oxide
B) acidic oxide
C) neutral oxide
D) amphoteric oxide
Answer:
B) acidic oxide
Question 8.
Match the following.
Group – A Group – B
1. Making into thin sheets ( ) A) Ductility
2. Shining materials ( ) B) Conductivity
3. Making into wires ( ) C) Sonority
4. Transmission of heat ( ) D) Lustrous
5. Making ringing sound ( ) E) Malleability
Answer:
1. E
2. D
3. A
4. B
5. C
Question 9.
Which gas makes a ‘pop’ sound if exposed to lighted matchstick?
Answer:
Hydrogen gas makes a pop sound when it is exposed to a lighted matchstick.
Question 10.
Why are bells made up of metals instead of wood
Answer:
Metals have sonority property that means they make ringing sound when we hit them with a hammer. Where wood does not give ringing sound when it is hit with a hammer. So bells are made up of metals instead of wood.
![]()
Question 11.
Imagine the human life without metals, write briefly about the consequences.
Answer:
- There would not be any utensil for cooking.
- There would not be trains, buses, aeroplanes for travelling.
- There would be no almirahs, no school bells, no agricultural material, no electrical appliances, no automobiles, no satellites, no machinery and no decorative material. So everything in life directly or indirectly depends upon metals. So we cannot imagine a life without metals.
Question 12.
After completion of metals and non-metals chapter, Raheem understood that metals are hard and non-metals are soft. During the discussion with his brother he came to know that diamond is a hardest material and it is a non-metal. Similarly mercury is a soft material and it is a metal. These findings from the discussion raised some questions in Raheem’s mind. Can you guess those questions? Write them.
Answer:
The doubts arised in Raheem’s mind are
- If diamond is hard how it behaves like a non-metal?
- If mercury is soft material how it acts as metal?
- What are the properties which actually decide whether the given material is either metal or non-metal?
Question 13.
Discuss the acidic and basic nature of the metals and non-metals with suitable experiments.
Answer:
Take a small strip of magnesium and burn it. It forms white ashes of magnesium oxide collect the ashes of magnesium and add some distilled water to it. Test the solution with red and blue litmus papers. Note the colour changes. The solution turns red litmus into blue. It indicates given solution is basic. So metallic oxides are basic in nature.
Take a small amount of powdered sulphur in a deflagrating spoon and heat it. As soon as sulphur starts burning, introduce the spoon into a gas jar or tumbler. Cover the tumbler with lid to ensure that the gas produced does not escape. The gas formed is sulphur dioxide. Remove the spoon after some time but try to keep the jar covered. Add a small quantity of water into the tumbler and quickly replace the lid. Shake the tumbler well. Check the solution with red and blue litmus paper. The solution turns blue litmus into red. It indicates the solution is acidic. So non-metallic oxides are acidic in nature.
![]()
Question 14.
How do you appreciate wide range utility of aluminium right from utensils to space craft?
Answer:
Aluminium is essential part of our life. Aluminium foil is used on inner packing of food materials and toffees. Aluminium and copper mixture is used in currency coins, medals and statues. Aluminium is used in electrical appliances, automobiles, satellites, space crafts, aeroplanes, cooking utensils, machinery, decorative materials. This indicates wherever we go we observe articles made up of aluminium. So we require aluminium for better living.
Question 15.
How is malleability of metals used in our daily life
Answer:
Metals can be flattened into sheets is called malleability. Due to this metals can be made into different shapes like railway coaches, railway tracks, cooking utensils, etc. So malleability of metals is extremely useful in daily life.
Question 16.
Dumping of waste material made up of metals and non-metals leads to environment pollution. Do you support the statement? Give your justification with suitable examples.
Answer:
Soil samples analyzed from location adjacent and with in deposite show high level of heavy metals in particular lead, mercury, cadmium, copper and chromium. In which lead and mercury are extremely harmful to environment as well as mankind. After medical evaluation the adolescents living and children living near dump site had high incidence of diseases that are associated with high exposure levels to these metal pollutants. So waste material made of metals and non-metals leads to environmental pollution. Some more examples are burning of sulphur forms sulphur dioxide and sulphur trioxide, which is a cause for acid rain and soil corrosion. In complete burning of carbon leads to release of poisonous carbon monoxide.
8th Class Physical Science 5th Lesson Metals and Non-Metals InText Questions and Answers
Think and Discuss
8th Class Physical Science Textbook Page No. 68
Question 1.
How will you close the circuit using sulphur, carbon or iodine? They may be in powder form. Try to tightly pack the powder in a straw and use it. Think of other ways!
Answer:
Circuit will not be completed by using non-metals like sulphur, carbon or iodine because they are bad conductors of electricity. So by packing the powder will not complete the circuit. There is no other by using which we can complete the circuit by using a non-metal.
![]()
Try This
8th Class Physical Science Textbook Page No. 74
Question 1.
Recall the names of the some of the laboratory acids and bases that you know. Write down their names in table and identify metal/non-metal present in them, which form oxides when react with oxygen. Take the help of your teacher.
Answer:
| Name of the base | Metal present in it | Name of the acid | Non-metal present in it |
| Calcium hydroxide (Ca(OH)2) | Calcium (Ca) | Sulphuric acid (H2SO4> | Sulphur (S) |
| Sodium hydroxide (NaOH) | Sodium (Na) | Hydrochloric acid (HCl) | Chlorine (Cl) |
| Magnesium hydroxide (Mg(OH)2) | Magnesium (Mg) | Nitric acid (HNO3) | Nitrogen (N) |
| Zinc hydroxide (Zn(OH2) | Zinc (Zn) | Phosphoric acid (H3PO4) | Phosphorus(P) |
| Sodium hydroxide (NaOH) | Sodium (Na) | Sulphurus acid (H2SO3) | Sulphur (S) |
| Cupric hydroxide (Cu(OH)2) | Copper (Cu) | Carbonic acid (H2CO3) | Carbon (C) |
Question 2.
Have you seen a periodic table?
Answer:
Yes.
Question 3.
Try to find the metals and non-metals that you come across in the chapter on the periodic table.
Answer:
Metals Non-metals
1) Zinc 1) Sulphur
2) Copper 2) Chlorine
3) Iron 3) Nitrogen
4) Potassium 4) Carbon
5) Sodium 5) Phosphorus
6) Gold
7) Silver
8) Magnesium
9) Calcium
10) Aluminium
8th Class Physical Science 5th Lesson Metals and Non-Metals Activities
![]()
Activity – 1
Question 1.
Observing appearance and colour of some materials:
Observe the appearance of your samples. Look at their colour. Decide whether they appear shining or dull and record your observations in table. If the surface seems dirty, clean it with sand paper.
| Sample | Appearance Shining / not shining | Colour |
| Iron | shining | grey |
| Zinc | shining | pale green |
| Copper | shining | red |
| Sulphur | not shining | yellow |
| Aluminium | shining | silver white |
| Carbon | not shining | black |
| Magnesium | shining | silver white |
| Iodine | shining | black |
a) Which of the samples did not shine even after you cleaned them with sand paper?
Answer:
Sulphur, carbon.
b) Generally metals are lustrous. Do all lustrous materials are metals?
Answer:
No. Some may be non-metals, e.g.: Iodine
c) We all know that mirror is lustrous. Can a mirror be called metal?
Answer:
No. Several properties required to decide if a given material is metal or not.
Activity – 2
Question 2.
Listening the sound produced by some material:

a) Drop a piece of coal on the floor and listen the sound.
Do you think coal is sonorous?
Answer:
No.
b) Take the pieces of zinc, copper, aluminium, magnesium and tightly packed packets of sulphur, carbon and iodine. Drop them one by one, on a hard surface. Listen carefully to the sound produced and record your observation in table.
Answer:
| Material sample that | Material sample that | |
| produce sound | do not produce sound | |
| Zinc | Sulphur | |
| Copper | Carbon | |
| Aluminium | Iodine | |
| Magnesium | ||
c) What similarity do you notice among materials which produce sound?
Answer:
All of them are metals.
d) Which property of metals first attracted the attention of human beings?
Answer:
Metals had the advantage of not just being harder but they could be heated in a fire and moulded or cast into different shapes. This property of metals first attracted the attention of human beings.
e) Do you bring a similar change in the shape of a clay material by beating it?
Answer: Yes, we bring similar change in the shape of a clay material by beating it.
![]()
Activity – 3
Question 3.
Identifying malleability of material:
Take a hammer and beat the material samples which are used in Activity-2 and observe the changes in material samples. Record your observations in the table.
Answer:
| Observing the change | Name of sample |
| Flattens | Iron, zinc, copper, aluminium |
| Breaks/ converts into powder | Magnesium, sulphur, carbon, iodine |
| No change | — |
Some of the samples like zinc, copper are flattened whereas some materials such as carbon, iodine broken into pieces.
The materials which can be flattened into thin sheets are called malleable materials.
Ductility:
We use wires in different situations in our daily life. Look at the samples given in the table.
a) Have you ever seen the wires made up of materials mentioned in the above table?
Answer:
Yes. I have seen the wires made of iron, copper, aluminium.
b) What is the property of drawing material to make wire is called?
Answer:
Ductility.
Activity – 4
Question 4.
Identifying electric conductivity of a material:
Arrange an electric circuit with a battery and bulb. Close the circuit using an iron nail, as shown in figure. Observe whether the bulb glows or not. Record your observations in table. Repeat the same experiment using the other samples and record your observations in the same table.
| Sample | Can we convert it into wires |
| Iron | Yes |
| Zinc | Yes |
| Copper | Yes |
| Sulphur | No |
| Aluminium | Yes |
| Carbon | No |
| Magnesium | No |
| Iodine | No |
a) Do all the samples allow the bulb to glow?
Answer:
No. Materials like iron, zinc, copper allows bulb to glow.
b) What is name given to these material which allows electricity to pass them and make to bulb to glow are called?
Answer:
They are called conductors.
c) Give examples for good conductors of electricity?
Answer:
Copper, iron, aluminium
d) Talk to an electrician. Look at the handles of his tools. Are the handles made of the same materials? If not why?
Answer:
No. The handles of his tools are made of electrical insulating material in order to avoid electrical shock.
![]()
Activity – 5
Question 5.
Observing heat conduction by metals:
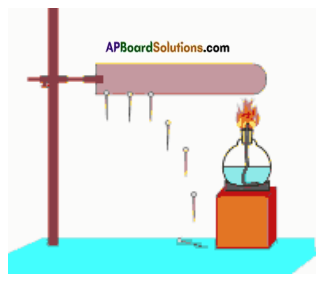
Take an iron rod. Stick pins on it with the help of wax. Now fix the rod to a stand as shown in figure. Heat one end of the rod with a spirit lamp and see how the pins fall off?
a) Why did the pins fall off from iron rod?
Answer:
Iron rod gains heat from spirit lamp.
b) Pin of which end fell off first?
Answer:
The pin which is near to spirit lamp falls first.
c) What could be the reason for this?
Answer:
The pin falls off because of heat supplied to the iron rod and makes the wax to melt at one end. The wax closer to the flame melted first and the pin falls off. Then the other pins falls. This activity shows that heat moves from one end of the rod to the other. This property of a material is known as conductivity of heat.
d) Go back to the list of samples. On the basis of all the activities carried out, fill the following table.
Answer:
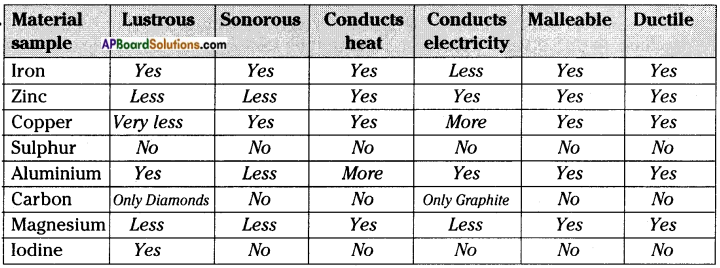
![]()
Lab Activity
Conduct an experiment to know the reaction of oxygen with metals and non-metals.
Answer:
Aim: To know reaction of oxygen with metals and non-metals.
Materials required: One metal sample (Magnesium) and one non-metal sample (Sulphur), spirit lamp or Bunsen burner and litmus paper, etc.
Procedure:
Take a small strip of magnesium and burn it. It burns brilliantly and produce white ashes of magnesium oxide due to reaction between magnesium and oxygen. Collect the ashes of magnesium and add some distilled water to it. Test the solution with red and blue litmus papers. Note the changes in table.
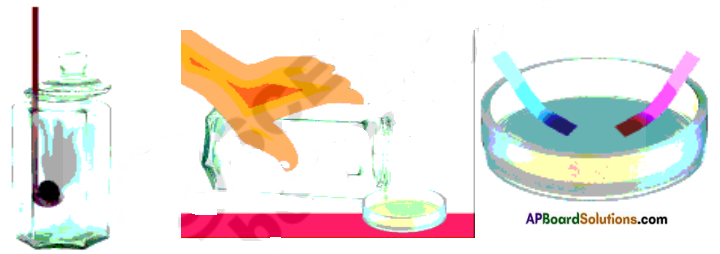
Take a small amount of powdered sulphur in a deflagrating spoon and heat it. Sulphur starts burning and forms sulphur dioxide. Introduce the spoon into a jar and cover the jar with a lid to ensure that the gas produced does not escape. Add small quantity of water into the jar quickly replace the lid. Shake the jar well. Check the solution with blue and red litmus papers. Record the changes in table.
| Sample | Physical Appearance before reaction | Physical Appearance after reaction | Effect of Litmus Paper |
| Magnesium | Silver white sheet | White ash | Turns red litmus into blue |
| Sulphur | Yellow powder | Colourless gas | Turns blue litmus into red |
From the table we can observe metallic oxide solutions are basic in nature whereas non metallic oxide solutions are acidic in nature.
Activity – 6
Question 6.
Experiment about reaction of metals with water.
Answer:
Take a 500 ml beaker or a big trough and fill half of it with water. Cut a small piece of sodium (which is kept in kerosene) and put the sodium piece in water using forceps. This piece of sodium floats on the surface of water with a hissing sound.
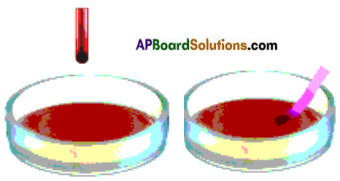
This shows that sodium is reacting extremely fast with water.
Repeat the same experiment using aluminium, and iron. We will not see any change even after five minutes. This is because these metals react extremely slow with water. This shows metals react with water at different rates.
![]()
Activity – 7
Question 7.
Reaction with Acids.
Fill the reactions of the following metals and non-metals with dilute hydrochloric acid and dilute sulphuric acid.
Iron, Zinc, Copper, Sulphur, Aluminium, Graphite, Magnesium and Iodine.
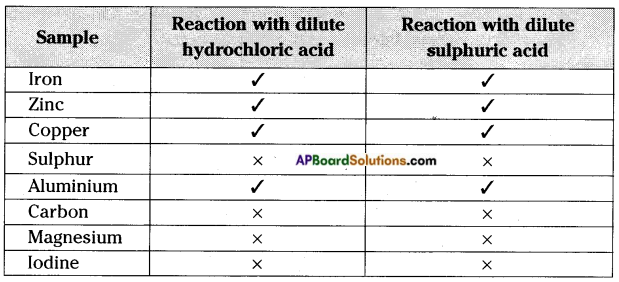
Observation:
We find that some metals react with dilute HCl or H2SO4 liberating hydrogen but non-metals usually do not react with acids.
Activity – 8
Question 8.
Reactivity of metals:
Take six beakers and label them a, b, c, d, e and f. Take 50 ml of water in each beaker and dissolve a spatulaful of copper sulphate in beakers ‘a’ and ‘b’. Dissolve a spatulaful of zinc sulphate, in beakers ‘c’ and ‘d’. Iron sulphate in beakers ‘e’ and ‘f’.

- Zinc granules in beaker ‘a and e’
- Iron nail in beaker ‘b and d’
- Copper turnings in beaker ‘c and f’
Leave the beakers undisturbed. Record the changes in the colour of the solution in the table.
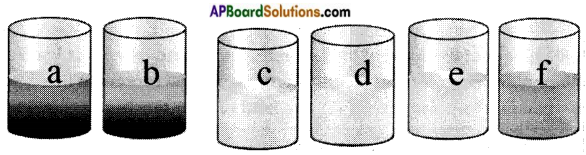
| Solutions | Observations |
| Beaker a | The blue colour of copper sulphate disappears and a powdery red mass of copper is deposited at the bottom of the beaker ’a’. |
| Beaker b | In beaker ’b’ red copper is found at the bottom of the beaker and on the nail leaving light green iron sulphate solution. |
| Beaker c | No change |
| Beaker d | No change |
| Beaker e | No change |
| Beaker f | No change |
a) What could be the reasons behind these changes?
Answer:
In beaker ‘a’, zinc displaces copper from copper sulphate giving rise to colourless zinc sulphate. Iron displaces copper from copper sulphate in beaker ’b’ leaving light green colour of iron sulphate and zinc displaces iron from Iron sulphate by change the colour from light green to colour less:
Copper sulphate + Zinc → Zinc sulphate + Copper
Copper sulphate + Iron → Iron sulphate + Copper
Iron sulphate + Zinc → Zinc sulphate + Iron
![]()
b) Do you find any changes in beakers c, d and f?
Answer:
There is no change in beakers c, d and f. The reason is
- Copper is unable to displace zinc from zinc sulphate in beaker ‘c’.
- Copper is unable to displace iron from iron sulphate in beaker ‘f’.
- Iron is unable to displace zinc from zinc sulphate in beaker ‘d’.
c) Why does iron is unable to displace zinc from zinc sulphate?
Answer:
Iron is less reactive than zinc. A more reactive metal can replace a less reactive metal from its salt solution. But a less reactive metal unable to displace a more reactive metal from its salt solution.
The order of reactivity of given metals are Zinc > Iron > Copper