Students get through AP Inter 2nd Year Chemistry Important Questions Lesson 6(b) Group-16 Elements which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions Lesson 6(b) Group-16 Elements
Very Short Answer Questions
question 1.
Write any two compounds, in which oxygen shows an oxidation state different from -2. Give the oxidation states of oxygen in them.
Answer:
OF2 and O2 F2 are two compounds in which oxygen shows an oxidation state different from -2.
- In OF2 the oxidation state of oxygen is +2.
- In O2F2 the oxidation state of oxygen is +1.
Question 2.
Why H2O a liquid while H2S Is a gas? ( IPE May – 2014)
Answer:
H2O is liquid due to the presence of intermolecular hydrogen bonding. While H2S is gas because it is not having such type of bonding.
![]()
Question 3.
H2O is neutral while H2S is acidic — explain.
Answer:
H2O is neutral while H2S is acidic.
Reason: The O-H bond dissociation enthalpy is greater than the S — H bond dissociation enthalpy.
Question 4.
Explain the structures of SF4 and SF6.
Answer:
Structure of SF4:
- In SF4 ‘S’ undergoes sp3d hybridisation.
- It has trigonal bipyramidal structure in which one of the equitorial positions is occupied by a lone pair of electrons. This geometry is also known as see – saw geometry.
Structure of SF6: - In SF6, ‘S’ undergoes sp3d2 hybridisation,
- It has octahedral structure.
Question 5.
Give one example each for
a) a neutral oxide
b) a peroxide
c) a super oxide
Answer:
a) CO, N2O are neutral oxides.
b) Na2O2, BaO2 are peroxides.
c) KO2, RhO2 are super oxides.
![]()
Question 6.
What is tailing of mercury? How is it removed? (IPE Mar – 2015 (TS))
Answer:
Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury
2Hg + O3 → Hg2O + O2
It is removed by shaking it with water which dissolves Hg2O.
Question 7.
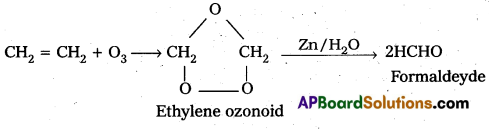
How does ozone react with Ethylene?
Answer:
Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
Question 8.
Which form of sulphur shows paramagnetism?
Answer:
In vapour state sulphur partly exists as S2 molecule which has two unpaired electrons in the antibonding π (π*)orbitals like O2. Hence it exhibits paramagnetism.
Question 9.
Why are group — 16 elements called chalçogens ?
Answer:
Chalcogens means mineral forming (or) ore forming elements. Most of elements exist in earth crust as oxides, sulphides, selinides, telurids etc. So Group – 16 elements are called as chalcogens.
Question 10.
Write any two uses each for O3 and H2SO4.
Answer:
Uses of O3:
- Ozone is used in sterilisation of water.
- Ozone is used in manufacture of artificial silk and camphor etc.
- Ozone is used to identify unsaturation in carbon compounds.
Uses of H2SO4:
- H2SO4 is used in the manufacture of fertilisers.
- H2SO4 is used in petrol refining.
- H2SO4 is used in detergent industry.
![]()
Short Answer Questions
Question 1.
Write a. short note on the allotropy of sulphur.
Answer:
The important allotropes of sulphur are
a) yellow rhombic (α. sulphur).
b) Monoclinic (β – sulphur).
The stable from is α-sulphur (at room temperature).
Rhombic sulphur (α – Sulphur):
- Colour : Yellow.
- Melting point : 3858K.
- Specific gravity: 2.06.
- It is insoluble in water and partially soluble in alcohol, benzene etc., and readily soluble in CS2.
Monoclinic sulphur (β – Sulphur):
- Melting point: 392K
- Specific gravity: 1.98.
- It is soluble in Cs2.
- Rhombic sulphur transforms to monoclinic sulphur by heating above 369K. This temperature is called transition temperature.
Question 2.
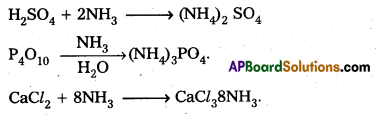
Which is used for drying ammonia?
Answer:
For drying ammonia quick lime (CaO) is used.
- For drying ammonia conc. H2SO4 , P4O10 and anhydrous CaCl2 cannot be used because they react with ammonia and forms (NH4)2SO4, (NH4)3PO4 and CaCl2. 8NH3
Question 3.
Explain the conditions favourable for the formation of SO3 from SO2 in the contact process of H2SO4.
Answer:
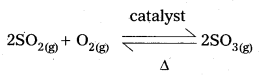
Le Chatlier’s principle — Application to produce SO3:
The oxidation of SO2 to SO3 in the presence of a catalyst is a reversible reaction. The thermochemical equation for the conversion is written as
![]()
The equation reveals the following points:
- 3 volumes of the reactants convert into 2 volumes of SO3. i.e., a decrease of volume accompanies the reaction.
- the reaction is an exothermic change.
- the catalyst may be present to increase the SO3 yields.
According to Le Chattier’s principle,
i. a decrêase in volume of the system is favoured at high pressures. But in practice only about 2 bar pressure is used. The reason for not using high pressures is acid resisting towers which can withstand high pressures cannot be built.
ii. exothermic changes are favoured at low temperatures. It is not always convenient in the industry to work at low temperatures. In such situations an optimum temperature is maintained. At this temperature considerable amounts of the product are obtained, in the manufacture of H2SO4, the optimum temperature suitable for the conversion of SO2 into SO3 is experimentally found to be 720K.
iii. The rate of formation of SO3 is enhanced by the use of a catalyst. (V2O5 (or) Pt – asbestos).
Favourable Conditions:
Temperature: 720K
Pressure :2 bar
Catalyst : V2O5 (or) platinized asbestos.
Question 4.
Which oxide of sulphur can act as both oxidizing and reducing agent? Give one example each.
Answer:
Sulphur dioxide (SO2) acts as both oxidising as well as reducing agent.
SO2 as Oxidising agent:
Sodium sulphide oxidises to hypo with SO2.
2Na2S + 3SO2 → 2Na2S2O3 + S
SO2 as Reducing agent:
SO2 reduces Fe+3 ions to Fe+2 ions.
2Fe+3 + SO2 + 2H2O → 2Fe2+ + \(\mathrm{SO}_4^{-2}\) + 4H+
![]()
Long Answer Questions
Question 1.
Explain in detail the manufacture of sulphuric acid by contact process. ( IPE 2016 (TS))
Answer:
Manufacture of H2SO4 by contact process:
Manufacturing of H2SO4 involves three main steps.
Step-I:
SO2 production : The required SO2 for this process is obtained by burning S(or) Iron
pyrites in oxygen.
S + O2 → SO2
4FeS2 + 15O2 → 2Fe2O3 + 8SO3
Step—2
SO3 formation: SO2 is oxidised in presence of catalyst with atmospheric air to form SO3.
Le Chatliers principle — Application to produce SO3 ;
The oxidation of SO2 to SO3 in the presence of a catalyst is a reversible reaction. The
thermochemical equation for the conversion is written as
![]()
The equation reveals the following points:
- 3 volumes of the reactants convert into 2 volumes of SO3. i.e., a decrease of volume accompanies the reaction.
- The reaction is an exothermic change.
- The catalyst may be present to increase the SO3 yields.
According to Le Chatlier’s principle,
- a decrease in volume of the system is favoured at high pressures. But in practice only about 2 bar pressure is used. The reason for not using high pressures is acid resisting towers which can withstand high pressures cannot be built.
- exothermic changes are favoured at low temperatures. It is not always convenient in the industry to work at low temperatures. in such situations an optimum temperature is maintained. At this temperature considerable amounts of the product are obtained. In the manufacture of H2SO4, the optimum temperature suitable for the conversion of SO2 into SO3 is experimentally found to be 720K.
- The rate of formation of SO3 is enhanced by the use of a catalyst. (V2 O5 (or) Pt – asbestos).
Favourable Conditions:
Temperature: 720K :
Pressure : 2 bar
Catalyst : V2O5 (or) platinized asbestos.
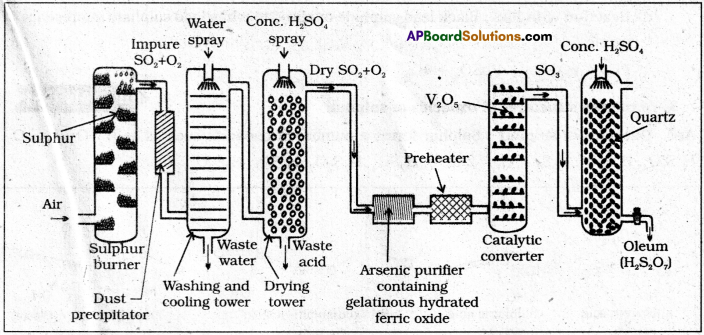
Step-3
- Formation of H2SO4: SO3 formed in the above step absorbed in 98% H2SO4 to get oleum (H2S2O7). This oleum is diluted to get desired concentration of H2SO4.
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → H2SO4
![]()
Question 2.
How is ozone prepared from oxygen? Explain its reaction with
a) C2H4
b) KI
c) Hg
d) PbS. (T.S. & A.P. Mar. ’17) (A.P.Mar. ’16) (Mar. ’14)
Answer:
Preparation of Ozone:
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3 ΔH° = 142kJ/mole .
- The formation of ozone is an endothermic reaction.
- It is necessary to use silent electric discharge in the preparation of O3 to prevent its decomposition
a) Reaction with C2H4 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to for formaldehyde.
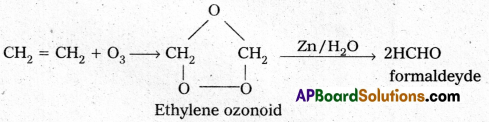
b) Reaction with KI : Moist KI is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
c) Reaction with Hg: Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
It is removed by shaking it with water which dissolves Hg2O.
d) Reaction with PbS : Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2.
Question 3.
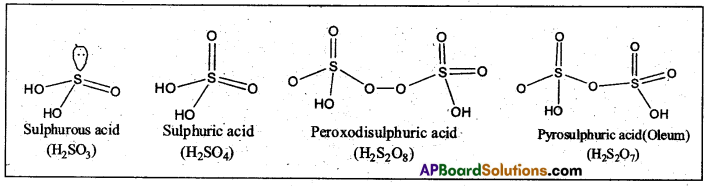
Write the structures of oxoacids of sulphur. (IPE 2016 (TS))
Answer:
Oxoacids of sulphur: Sulphur forms a number of oxoacids such as H2SO3. H2S2O3, H2S2O4, H2S2O5, H2S2O6 (x = 2 to 5), H2SO4, H2S2O7, H2SO5, H2S2O8.
Question 4.
Write any two oxidation and any two reduction properties of ozone with equations.
Answer:
Oxidation properties:
- Ozone oxidises moist potassium idodide and liberates I2.
2KI + H2O + O3 → 2KOH + I2 + O2 - Ozone oxidises black lead sulphide to white lead sulphate. PbS + 4O3 → PbSO4 + 4O2
Reduction properties:
- Ozone reduces H2O2 to H2O. H2O2 + O3 → H2O + 2O2
- Ozone reduces Ag2O to Ag. Ag2O + O3 → 2Ag + 2O2