Students can go through AP State Board 10th Class Physical Science Notes Chapter 6 Structure of Atom to understand and remember the concept easily.
AP State Board Syllabus 10th Class Physical Science Notes Chapter 6 Structure of Atom
→ Light can be characterized by its wavelength and frequency (v) and these quantities related to the speed of light as c = vλ
→ A spectrum is a group of wavelengths.
→ Electromagnetic energy (light) can have only certain discrete energy values which are given by the equation E = hv.
→ Electrons in an atom can gain energy by absorbing a particular frequency of light and can lose energy by emitting a particular frequency of light.
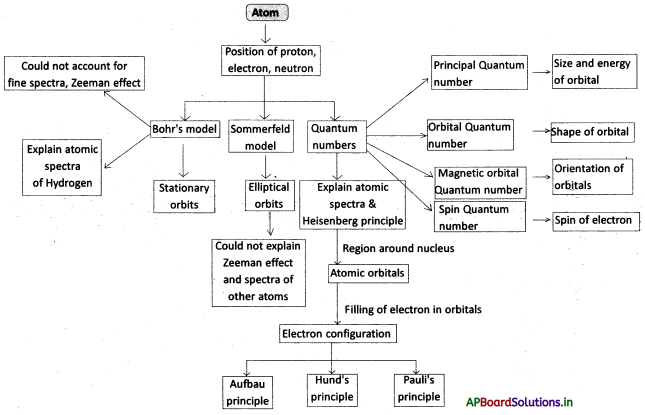
→ Bohr’s model of an atom: Electrons are present in stationary states. The electron moves to a higher energy level If it absorbs energy in the form of electromagnetic energy or moves to a lower energy state by emitting energy in the form of electromagnetic energy of appropriate frequency.
→ Atomic line spectra arise because of the absorption/emission of certain frequencies of light energy.
→ It is not possible to measure the position and velocity of an electron simultaneously.
![]()
→ The space around the nucleus where the probability of finding electrons is maximum is called orbital.
→ The three quantum numbers n, l, m describe the energy, shape and orientation respectively of an atomic orbital.
→ Spin is an intrinsic property of an electron.
→ The arrangement of electrons in shells, sub-shells and orbitals in an atom is called the electron configuration.
→ According to Pauli’s exclusion principle, no two electrons of the same atom can have the entire four quantum numbers same.
→ Aufbau principle: The lowest energy orbitals are filled first.
→ Hund’s rule: The orbitals of equal energy (degenerate) are occupied with one electron each before the pairing of electrons starts.
→ As long as an electron revolves in a stationary orbit it neither loses nor gains energy.
→ A rainbow is a natural spectrum appearing in the sky.
→ Light is considered an electromagnetic wave.
→ Electromagnetic waves are produced when an electric charge vibrates.
→ The electromagnetic wave has a speed of light, i.e. 3 × 108 ms-1.
→ The relation between frequency and velocity of light is c = vλ.
→ An atom or molecule having the lowest possible energy is said to be in-ground state otherwise it is said to be in an excited state.
→ An atom or molecule in an excited state can emit light to lower its energy in order to get stability.
→ Sodium vapour lamp emits light of the wavelengths 589.0 nm to 589.6 nm.
→ Line spectrum is also known as discrete spectrum.
→ The atomic spectrum of a hydrogen atom is a line spectrum.
→ Stationary states are also called energy levels.
→ Sommerfeld proposed elliptical orbits.
→ The Quantum mechanical model of an atom was developed by Erwin Schrodinger.
![]()
→ The principal quantum number explains the size.
→ As n increases, the orbitals become larger and the electrons in those orbitals are farther from the nucleus.
→ The number of electrons in a shell is 2n2.
→ Orbital quantum number (l) gives the shape of the orbital.
→ l takes values from 0 to n – 1.
→ The splitting of spectral lines in a magnetic field is called the Zeeman effect.
→ The splitting of spectral lines in an electric field is called the Stark effect.
→ The orientation of the orbital with an external magnetic field determines the magnetic orbital quantum number.
→ The magnetic orbital quantum number takes values from – l to l.
→ Orbitals of the same energy are called Degenerate orbitals.
→ Wave: Surging movement of water, electricity, etc.
→ Spectrum: A spectrum is a group of wavelengths or frequencies.
→ Intensity: The amount of energy per unit volume is called intensity.
→ Discrete energy: Definite energy s called discrete energy.
→ Line spectrum: The colours correspond to certain discrete wavelengths of light and are called Line spectrum. It Is also known as discrete spectrum.
→ Orbital: The region of space around the nucleus where the probability of finding an electron is maximum is called an orbital.
→ Quantum numbers: The numbers indicate the probability of finding the electron in the space around the nucleus.
→ Shell (or) orbit: The path of an electron around the nucleus is called shell or orbit.
→ Sub-shells: Atomic orbitals are also called sub-shells.
![]()
→ The shape of orbital electron spin: Electron has two types of spins. One is clockwise spin and the other is anti-clockwise spin,
→ Electronic configuratIon: The distribution of electrons in various atomic orbitals called electronic configuration.
→ Pauli’s exclusion principle: No two electrons of the same atom can have all four quantum numbers the same.
→ Aufbau principle: The electrons should be placed in the lowest available orbital until the total number of electrons added is equal to the atomic number.
→ Hund’s rule: This rule states that electron pairing in orbitals starts only when all available empty orbitals of the same energy are singly occupied.
→ Dispersion: The splitting of light into different colours (VIBGYOR) is called dispersion.
→ Electromagnetic wave: When an electric field and magnetic field are perpendicular to each other and at right angles to the direction of propagation of the wave is formed. Such a wave is called an electromagnetic wave.
→ Electromagnetic spectrum: The entire, range of electromagnetic wave frequencies is known as the electromagnetic spectrum.
→ Emission spectrum: When a light beam emitted by a source ¡s dispersed to get the spectrum Is called an emission spectrum.
→ Continuous spectrum of emission: When light is dispersed from a source spectrum of continuously distributed colours is obtained on a dark background is called a continuous spectrum of emission.
→ Line spectrum of emission: When a light ¡s dispersed with a sharp bright line on a dark background, such a spectrum is called a tine spectrum of emission.
→ Stationary orbits: Orbits of fixed energy are called stationary orbits of energy levels.
→ Heisenberg’s Principle of Uncertainty: It is not possible to find the exact position and velocity of an electron simultaneously and accurately.
→ Principal Quantum Number (n): The quantum number which explains the size and energy of orbitals is called principal quantum number.
→ Angular Momentum Quantum Number (l): The quantum number which defines the shape of the orbital occupied by the electron and the orbital angular momentum of the electron in motion (m).
→ Magnetic orbital quantum number (ml): The orientation of orbital with an external magnetic field determines the magnetic orbital quantum number.
![]()
→ Spin quantum number (s): It gives spin of the electrons about their own axis.
→ c = vλ: The frequency (v), wavelength (λ) and velocity of light (c) related as follows c = vλ
→ E = hv: Electromagnetic energy can have only certain discrete energy values which is given by the equation E = hv where h = Planck’s constant
v = frequency of radiation
→ Visible spectrum: The spectrum which visible to our eyes s called the visible spectrum.
→ Duplet configuration: ns2 configuration is called duet configuration.
→ Octet configuration: If the element has eight electrons in the outermost orbital, It is called Octet configuration.
→ Zeeman effect: Splitting of spectral lines in a magnetic field is called the Zeeman effect.
→ Stark effect: Splitting of spectral lines In electric field Is called Stark effect.
→ Degenerate orbitals: OrbitaIs of the same energy is called degenerate orbitals.
→ Prince Louis Victor DeBroglie:
- de Broglie, a French theoretical physicist born in 1892, received the Nobel Prize in 1929 ¡or his work on the wave nature of electrons.
- He proposed dual nature 01 electron which is very much used in the determination of characterisation 01 electron and structure 0f atom.
- He gave the wavelength (λ) material wave as I = \(\frac{\mathbf{h}}{\mathbf{m v}}\)
![]()
→ Neils Henrik David Bohr
- Neils Henrik David Bohr was a Danish physicist.
- He made foundational contributions to understanding atomic structure and quantum theory.
- He received the Nobel Prize in Physics for those foundational contributions in 1522.
- Bohr was also a philosopher and a promoter.
→ Max Karl Ernst Ludwig Planck:
- Max Karl Ernst Ludwig Planck was a German theoretical physicist.
- He originated quantum theory, which won him the Nobel Prize n Physics in 1918.
- Planck made many contributlon6 to Theoretical physics, but his lame rests primarily on his role as originator of the quantum theory.
- This theory revolutionized human understanding of atomic and subatomic processes.