Students can go through AP State Board 10th Class Physical Science Notes Chapter 7 Classification of Elements- The Periodic Table to understand and remember the concept easily.
AP State Board Syllabus 10th Class Physical Science Notes Chapter 7 Classification of Elements- The Periodic Table
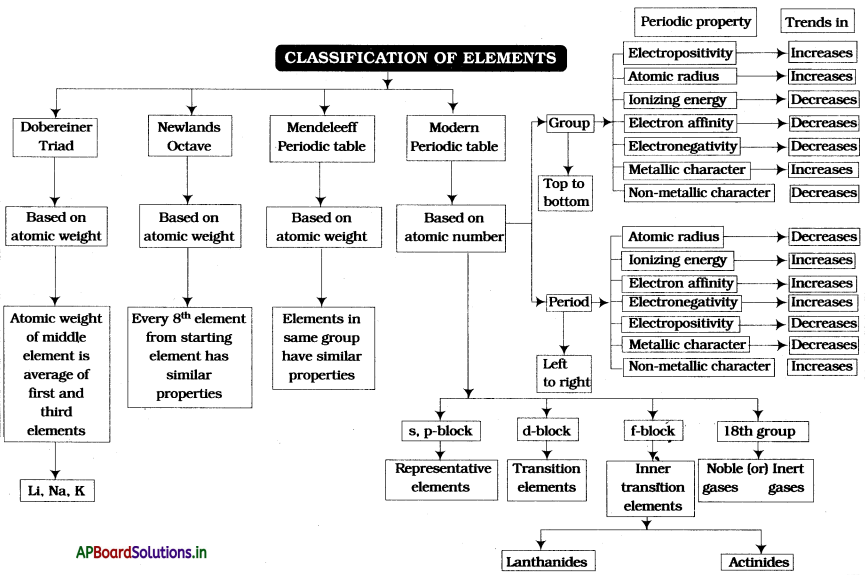
→The group of three elements in ascending order of their atomic weights in which the molecular weight of a middle element is the average of the first and third element is called a triad.
→ Elements are arranged in ascending order of their atomic weights, then every eighth element from starting element has similar properties. This is called an octave.
→ The horizontal rows and vertical columns of a periodic table are called periods and groups respectively.
→ The systematic arrangement of elements in a table in horizontal rows and vertical columns is called a periodic table.
→ Mendeleev’s periodic law: The physical and chemical properties of the elements are periodic functions of their atomic weights.
→ Atomic weight = Equivalent weight × valency.
→ Moseley’s periodic law: The physical and chemical properties of elements are periodic functions of their atomic numbers.
→ If differentiating electron enters s sub-shell, they are called s-block elements.
→ If differentiating electron enters p, d, and f shells they are called p-block, d-block, and f-block elements respectively.
→ The Group of elements is called the element family or chemical family.
→ Elements having atomic numbers 58 to 71 are called lanthanides.
→ Elements having atomic numbers 90 to 103 are called actinides.
→ Metalloids or semi-metals are elements that have properties that are intermediate between the properties of metals and non-metals.
→ Elements are classified on the basis of similarities in their properties.
→ Dobereiner grouped the elements into Triads and Newlands gave the law of octaves.
![]()
→ Modern periodic law: The physical and chemical properties of the elements are periodic functions of their electronic configurations.
→ Anomalous in the arrangement of elements based on increasing atomic mass could be removed when the elements were arranged in order of increasing atomic number, a fundamental property of the element discovered by Moseley.
→ Elements in the modern periodic table are arranged in 18 groups and 7 periods.
→ Elements are classified into s, p, d, f blocks depending upon which sub-shell the differentiating electron enters in the atom of the given element.
→ All the d-block elements (except the Zn group) are known as transition elements and all the f-block elements (both Lanthanides, Actinides) are known as inner transition elements.
→ Half of the distance between nuclei of two atoms in solids is called atomic size.
→ Atomic size is measured in a picometer.
1 pm = 10-12 m.
→ Atomic radii of metals are called metallic radii.
→ The half of the distance between the two atoms in a covalent molecule is called the covalent radius.
→ The size of the positive ion is less than the neutral atom.
→ The size of the negative ion is greater than the neutral atom.
→ The energy required to remove an electron from the outermost orbit or shell of a neutral gaseous atom is called ionization energy (or) first ionization energy.
![]()
→ The energy required to remove the electron from unipositive ion is called 2nd ionization energy.
→ The electron affinity of an atom is defined as the energy liberated when an electron is added to its neutral gaseous atom.
→ The energy liberated when an electron is added to a uni-negative ion is called the 2nd electron affinity of that element.
→ The relative tendency of its atom to attract electrons towards itself when it is bounded to the atom of another element is called electronegativity of the element.
→ Triad: A group of three elements in which the atomic weight of a middle element is the average of first and third elements with similar properties.
→ Octave: When elements are arranged in the ascending order of their atomic weight, every element starting from a given element resembles in its properties to that of the starting element are called octaves.
→ Periodic Law: Law that regards the properties of elements in the periodic table.
→ Periodic table: The charts of elements arranged ¡n a systematic order of increasing order of atomic weight or atomic number is called periodic table,
→ Periods: The horizontal rows in the periodic table are called periods.
→ Groups: The vertical columns in the periodic table are called groups.
→ Lanthanides: Elements from 58Ce to 71Lu possess almost the same properties as 57La. So they are called lanthanides.
![]()
→ Actinides: Elements from 90Th to 103Lr are called actinides.
→ Element family: The elements that belong to the same group in the periodic table having similar properties are called element families.
→ Metalloids: The elements which have both metallic and non-metallic properties are called metalloids.
→ Periodicity: The properties of elements in a group of a periodic table have similar properties, that is called periodicity.
→ Atomic radius: The half of distance between two nuclei in solids and half of distance between two atoms participated in a chemical bond. Simply we can give the distance between the nucleus and outermost orbital.
→ Ionization energy: The energy required to remove an electron from the outermost orbit or shell of a neutral gaseous atom is called ionization energy.
→ Electron affinity: The electron affinity of an element is the energy liberated when an electron is added to a neutral gaseous atom.
→ Electronegativity: The relative tendency of the element of an atom to attract electrons towards itself when it is bonded to another atom of another element.
→ Electropositivity (or): The tendency of metals to lose electrons is called Electro- Electropositive character positive character.
→ Mendeleev’s periodic law: The properties of elements are the periodic functions of their atomic weights.
→ Modern periodic law: The properties of elements are the periodic functions of their atomic numbers.
→ Alkali metal family: Group IA elements are called the alkali metal family.
→ Alkali earth metal family: Group lIA elements are called the alkali earth metal family.
![]()
→ Chalcogen family: The elements in group (16) form ores with elements.
→ Noble gases: The elements of the group (18) (VIII-A) which are least active.
→ Electron gain enthalpy: Electron affinity of the element is also called electron gain enthalpy.
→ Transition elements: d-block elements are called transition elements.
→ Inner transition elements: Lanthanoids and Actinoids (f block elements) are called inner transition elements.
→ Metallic radius: Half of the distance between adjacent nuclei of atoms in solid.
→ Covalent distance: Half of the distance between atoms in a covalent bond.
→ Valence (or) Valency: The combining power of elements with respect to hydrogen or oxygen.
→ Metals: The elements with three or fewer electrons in the outer shell are called metals.
→ Non-metals: The elements with five or more electrons in the outermost shell are called non-metals.
![]()
→ Pico meter: The unit of atomic size.
1 pm = 10-12 m.
→ Dmitri Ivanovich Mendeleev (8 Feb. 1834 – 2 Feb. 1907):
- Dmitri Ivanovich Mendeleev was a Russian chemist and Inventor.
- He formulated periodic law, created his own version of the periodic table of elements.
- He used the periodic table to correct the properties of some already discovered elements.
- And also predict the properties of elements yet to be discovered