Students can go through AP State Board 9th Class Physical Science Notes Chapter 3 Is Matter Pure? to understand and remember the concept easily.
AP State Board Syllabus 9th Class Physical Science Notes Chapter 3 Is Matter Pure?
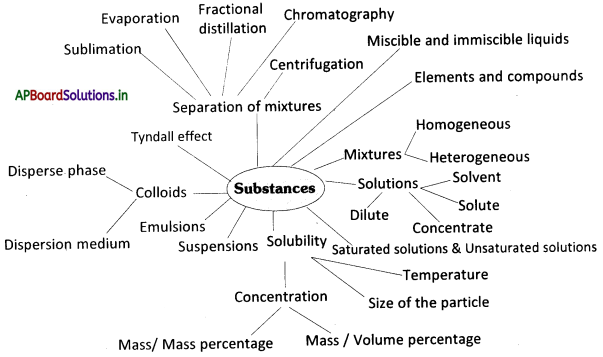
→ In our day-to-day language, pure means something with no adulteration.
→ The mixture is made up of two or more components that are not chemically combined.
→ Mixtures are homogeneous and heterogeneous.
→ The substance is homogeneous, when its composition doesn’t change, no matter which part of the substance we take for examination.
→ A heterogeneous mixture is a mixture made up of different substances, or the same substance in different stages which are not uniformly distributed in it.
→ The solution is a homogeneous mixture of two or more substances that we cannot separate by filtration.
→ The solution has two components i.e., solvent and solute.
→ The solvent is the substance that dissolves others in it.
→ The solute is the substance, that dissolved in the solvent,
![]()
→ An amount of solute present in a saturated solution at a certain temperature is called its solubility.
→ When no more solute can be dissolved in the solution at a certain temperature, it is said to be a saturated solution.
→ If the amount of solute contained in a solution is less than the saturation level, it is called an unsaturated solution.
→ A solution is said to be dilute if the amount of solute is little and concentrate if the amount of solute is large.
→ The concentration of a solution is the amount of solute in grams presents per 100 ml or per 100 g of the solution.
→ Materials that are insoluble in a solvent and have particles that are visible to naked eyes are called suspension. A suspension is a heterogeneous mixture.
→ A mixture of two liquids that do not mix and settle into layers when they are left undisturbed is called emulsion.
→ Colloids are heterogeneous mixtures in which the particle size is too small to be seen with the naked eye, but is big enough to scatter light.
→ Colloids consist of two phases: disperse phase and dispersion medium.
→ The scattering of a beam of light is called the Tyndall effect.
![]()
→ We can use different techniques like handpicking, sieving/filtration, flotation, crystallization, chromatography, sublimation, evaporation, distillation, fractional distillation, centrifugation, etc. to separate mixtures.
→ Pure substances can be elements or compounds.
→ An element is a form of matter that cannot be broken down into simpler substances by chemical reactions.
→ A compound is a substance composed of two or more different types of elements, chemically combined in a fixed proportion.
→ Properties of compounds are different from their constituent elements, whereas mixture shows the properties of its constituting elements or compounds.
→ Pure substance: A substance is said to be pure i.e., homogeneous when the composition doesn’t change, no matter which part of the substance you take for examination.
→ Mixture: A mixture is generally made of two or more components that are not chemically combined.
→ Heterogeneous: A heterogeneous mixture is a mixture made up of different mixture substances or the same substance in different states which are not uniformly distributed in it.
→ Homogeneous: In a homogeneous mixture the components of the mixture are mixture uniformly distributed throughout it.
→ Solution: ‘The homogeneous mixture of two or more substances is that we can not separate them by the process of filtration called a solution.
→ Suspension. Materials that are insoluble in a solvent and have particles that are visible to naked eyes, form suspension. A suspension is a heterogeneous mixture.
→ Colloids: These are heterogeneous in nature and always consist of at least two types of phases and scatter a beam of visible light.
![]()
→ Colloidal dispersions: Colloids are heterogeneous mixtures in which the particle size is too small to be seen with the naked eye but big enough to scatter light.
→ Solvent: The component of the solution that dissolves the other component in it (usually the component present in larger quantity) is called the solvent.
→ Solute: The component of the solution that is dissolved in the solvent, (usually the component present in lesser quantity) is called the solute.
→ Concentration: The amount of solute present in a given amount of solution
(or)
The amount of solute dissolved in a given volume of solution is called the concentration of the solution.
→ Tyndall effect: The scattering of a beam of light is called the Tyndall effect.
→ Evaporation: Evaporation is a technique of separation of mixtures like salt and water or sugar and water, etc.
→ Centrifuge: Centrifuge ¡s a machine used to separate the mixtures like cream from milk, etc.
→ Miscible liquid: A liquid is said to be miscible if it dissolves completely in another liquid.
→ Immiscible liquid: An immiscible liquid is one that doesn’t dissolve but forms a layer over another liquid and can be separated easily.
→ Chromatography: Chromatography is a laboratory technique for the separation of mixtures into their individual components like inks and dyes.
→ Distillation: Distillation is used in the separation of components of a mixture containing two miscible liquids whose boiling points have a large difference (greater than 25°C).
→ Fractional distillation: Fractional distillation process is used to separate two or more miscible liquids when the difference in their boiling points is less than 25°C.
→ Element: An element is a basic form of matter that cannot be broken down into simpler substances by chemical reactions.
→ Compounds: Compounds are pure substances that can be separated into two or more components only by means of a chemical reaction.
![]()
→ Disperse phase: It is the substance that presents in small proportion and consists of particles of the colloidal size of mm to 100 nm.
→ Dispersion medium: It is the medium in which the colloidal particles are dispersed.
→ Emulsion: Emulsion is a mixture consisting of two liquids, that do not mix and settle into layers when they are left undisturbed.
→ Humphry Davy:
- Humphry Davy was born on 17 December 1778 and died on 29 May 1829.
- He was an English chemist and inventor.
- He discovered potassium, sodium, and boron.
- He invented the Davy lamp.