Andhra Pradesh BIEAP AP Inter 1st Year Chemistry Study Material 2nd Lesson Classification of Elements and Periodicity in Properties Textbook Questions and Answers.
AP Inter 1st Year Chemistry Study Material 2nd Lesson Classification of Elements and Periodicity in Properties
Very Short Answer Questions
Question 1.
What is the difference in the approach between Mendeleev’s periodic law and the modern periodic law?
Answer:
- According to Mendeleev, elements’ physical and chemical properties are periodic functions of their atomic weights.
- According to modem periodic law, elements’ physical and chemical properties are periodic functions of their atomic numbers.
Question 2.
In terms of period and group, where would you locate the element with Z = 114?
Answer:
Element Z = 114 is present in 7th period and IVA group (Group – 14)
![]()
Question 3.
Write the atomic number of the element, present in the third period and seven¬teenth group of the periodic table.
Answer:
The Element present in 3rd period and Group – 17 (VIIA group) is chlorine (Cl). It’s atomic number is 17.
Question 4.
Which element do you think would have been named by
a) Lawrence Berkeley Laboratory .
b) Seaborg’s group
Answer:
a) Lawrence Berekeley Laboratory – Lanthanide
b) Seaborg’s group – Actinide (Transuranic element).
Question 5.
Why do elements in the same group have similar physical and chemical properties ?
Answer:
Elements in the same group have same no. of valency shell electrons and have similar outer elec¬tronic configuration so these have similar physical and chemical properties.
Question 6.
What are representative elements ? Give their valence shell configuration.
Answer:
- Representative elements are s and p-block elements except zero group.
- These have general electronic configuration ns1-2np1-5.
Question 7.
Justify the position of f-block elements in the periodic table.
Answer:
The two series of elements lanthanides and actinides have been grouped separately and placed at the bottom of the periodic table, though they belong to the sixth and seventh periods of third group (III B).
The justification for assigning one place to these elements has been given on the basis of their similar properties. The properties are so similar that elements from Ce to Lu can be considered as equivalent to one element. In case these elements are assigned different positions (i.e.,) arranged in order of their increasing atomic numbers, the symmetry of the whole arrangement would be disrupted. The same explanation can be given in the case of actinides.
Question 8.
An element ‘X’ has atomic number 34. Give its position in the periodic table.
Answer:
The element X with atomic no (z) = 34 has electronic configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p4. Hence the element is present in 4,h period and 16th group (VIA group).
Question 9.
What factors impart characteristic properties to the transition elements ?
Answer:
Transition elements exhibits characteristic properties because
- The differentiating electron enters into penultimate d-subshell
- These elements have small size.
- These possess high effective nuclear charge.
Question 10.
Give the outer shells configuration of d-block and f-block elements.
Answer:
- The outer shell electronic configuration of d-block – elements is ns1-2 (n-1)d1-10
- The outer shell electronic configuration of f-block – elements is ns2(n-1)d0 (or) 1 (n – 2) f1-14
![]()
Question 11.
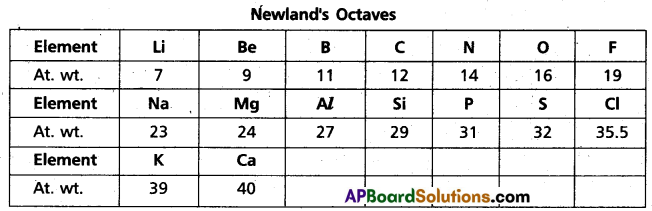
State and give one example for Dobereiner’s law of triads and Newland’s law of octaves.
Answer:
1) Dobereiner law :
- According to Dobereiner in a traid (3 – elements) the atomic weight of the middle element is the arithmatic mean of the other two elements.
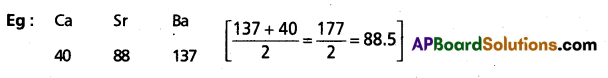
2) Newland’s law of octaves : According to Newlands, the elements arranged in the increasing order of atomic weights noticed that every eight element had properties similar to the first element. This relationship was just like every eight note that resembles the first in octaves of music.
Question 12.
Name the anomalous pairs of elements in the Mendaleev’s periodic table.
Answer:
In Mendeleev’s periodic table anamalous pairs are the elements whose atomic weights increasing order is reversed.
Eg:

Question 13.
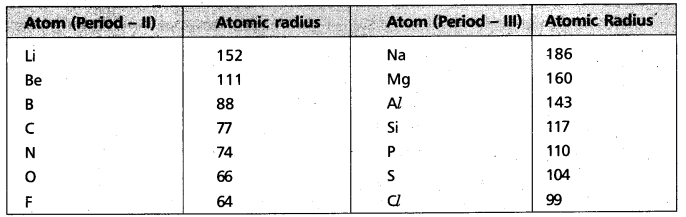
How does atomic radius vary in a period and in a group ? How do you explain the variation ?
Answer:
In a period : Atomic radius decreases generally from left to right in a period.
Reason : In periods electrons are entered into same subshells.
In a group : Atomic radius increases generally from top to bottom in a group.
Reason : In groups electrons are entered into new subshells.
Question 14.
Among N-3; O-2, F–, Na+, Mg+2 and Al+3
a. What is common in them ?
b. Arrange them in the increasing ionic radii.
Answer:
Given ions are
N-3, O-2, F–, Na+, Mg+2 and Al+3.
a) The above ions have same number of electrons (All have 10 electrons). So these are called iso electronic species.
b) The increasing order of ionic radii among above ions is
Al+3 < Mg+2 < Na+ < F– < O-2 < N-3
Reason : – In case of iso electronic species as the nuclear charge increases ionic radii decreases.
Question 15.
What is the significance of the term isolated gaseous atom while defining the ionization enthalpy.
Hint: Requirement for comparison.
Answer:
- Isolated gaseous atom’s ionisation enthalpy is taken as reference value and it is required to compare this values to various ions of this elements and to compare this values with various elements.
Question 16.
Energy of an electron in the ground state of the hydrogen atom is – 2.18 × 10-18J. Calculate the ionization enthalpy of atomic hydrogen in terms of J mol-1.
Answer:
Given that the energy of the electron in the ground state for hydrogen atom = – 2.18 × 10-18 J.
For 1 mole of atoms is given by – 2.18 × 10-18 J × 6.023 × 1023
= -13.13 × 105 J/Mole
∴ Ionisation enthalpy of hydrogen atom = 13.13 × 105 J/Mole.
Question 17.
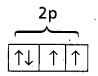
Ionization enthalpy, (IE1) of O is less than that of N — explain.
Answer:
- Oxygen has electronic configuration 1s2 2s2 2p4
- Nitrogen has electronic configuration 1s2 2s2 2p3
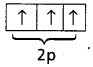
- Nitrogen has half filled shell and is stable so more amount of energy is required to remove an electron, than in oxygen.
Hence IE, of ‘O’ is less than that of ‘N’.
Question 18.
Which in each pair of elements has a more negative electron gain enthalpy?
a. O or Fb. F or Cl
Answer:
a)
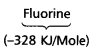
has more negative electron gain enthalpy than that of
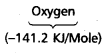
b)
![]()
has more negative electron gain enthalpy than that of
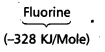
![]()
Question 19.
What are the major differences between metals and non-metals ?
Answer:
Metals
- These are generally in solid form (Except Hg)
- These are good conductors of heat and electricity.
- These have high m.pts and b.pts.
- Generally these are electropositive.
- These forms more ionic compounds.
Nón metals
- These may be solids (or) gases (or) liquids.
- These are not good conductors of heat and electricity.
- These have low m.pts and b.pts.
- Generally these are electronegative.
- These forms more covalent compounds.
Question 20.
Use the periodic table to identify elements.
a. With 5 electrons in the outer subshell
b. Would tend to lose two electrons
c. Would tend to gain two electrons.
Answer:
a) The elements possessing 5 electrons in the outer most shell are group 15 (VA) elements.
- General outer electronic configuration is ns2 np3 Eg. : N, P, As………
b) The elements tend to lose two electrons are Group — II elements.
- General outer electronic configuration is ns2 Eg: Mg, Ca, Sr etc.
c) The elements tend to gain two electrons are Group – VIA elements (16th group).
- General outer electronic configuration is ns2 np4 Eg : O, S, Se ………
Question 21.
Give the outer electronic configuration of s, p, d and f – block elements.
Answer:

Question 22.
Write the increasing order of the metallic character among the elements B, Al, Mg and K.
Answer:
Given elements are B, Al, Mg and K
The increasing order of metallic character is
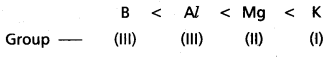
Question 23.
Write the correct increasing order of non – metallic character for B, C, N, F and Si.
Answer:
Given elements are B, C, N. F and Si
The increasing order of non- metallic character is
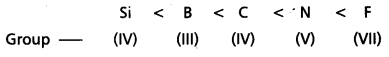
Question 24.
Write the correct increasing order of chemical reactivity in terms of oxidizing property for N, O, Fand Cl.
Answer:
The correct increasing order of chemical reactivity in terms of oxidizing property for N, O, F and Cl is F > 0 > Cl > N.
Question 25.
What is electronegativity ? How is this useful in understanding the nature of elements?
Answer:
Electronegativity : The tendency of an element (or) atom to attract the shared pair of electrons towards itself in a molecule is called electronegativity.
- On the basis of electronegativity values nature of elements can be predicted. Higher electro-negativity values indicates that element is non metal and lower values indicates that the element is a metal.
- On the basis of electronegativity values bond nature also predicted (Ionic/covalent).
Question 26.
What is screening effect? How is it related to lE?
Answer:
The decrease of nuclear attraction on outer most shell electrons due to presence of inner energy electrons is called screening effect.
- As the screening effect increases LE. values decreases.
![]()
Question 27.
How are electronegativity and metallic & non-metallic characters related?
Answer:
- Greater the electronegativity values of an element indicates that non metallic nature and low metallic nature of that element.
- Lower the electronegativity value of an element indicates that low non metallic nature and high metallic nature of that element.
Electronegativity ∝ Non metallic nature
Electronegativity ∝ \(\frac{1}{\text { Metallic Nature }}\)
Question 28.
What is the valency possible to Arsenic with respect to oxygen and hydrogen?
Answer:
- The valency of Arsenic with respect to hydrogen is ‘3’
Eg : AsH3 - The valency of Arsenic with respect to oxygen is ‘5’
Eg : As2O5
Question 29.
What is an amphoteric oxide? Give the formula of an amphoteric oxide formed by an element of group – 13.
Answer:
The oxide which contains both acidic as well s basic nature is called amphoteric oxide.
- The oxides reacts with both acids and bases and forms salts.
Eq : Al2O3 is one of the amphoteric oxide formed by the Group — 13 element Aluminium.
Question 30.
Name the most electronegative element. Is it also having the highest electron gain enthalpy? Why or Why not?
Answer:
The most electronegative element is fluorine (F).
- It doesnot have high electron gain énthalpy.
Reasons : —
- Due to small size
- Due to high inter electronic repulsions.
- Chlorine has high electron gain enthalpý.
Question 31.
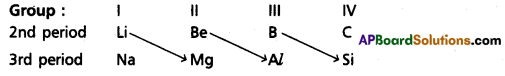
What is diagonal relation? Give one pair of elements, that have this relation.
Answer:
On moving diagonally across the periodic table the elements show certain similarities. An element of group in 2nd period has similar properties with second element of the next higher group in the 3rd period. This type of resemblance is called diagonal relationship. e.g. : (Li, Mg); (Be, Al); (B, Si)
Question 32.
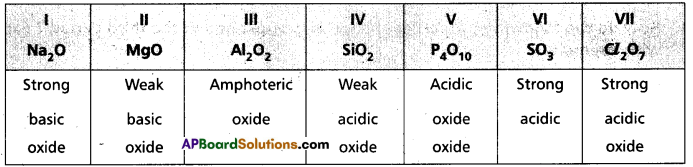
How does the nature of oxides vary in the third period?
Answer:
In 3rd period from left to right the oxide nature varies from high basic nature to high acidic nature.
- Basic nature gradually decreases and acidic nature gradually increases.
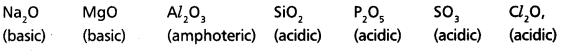
Question 33.
Radii of iron atom and its ions follow Fe > Fe2+ > Fe3+ – explain.
Answer:
When the positive charge on the ion increases, the effective nuclear charge on the outer electrons increases.
Hence the ionic size decreases in the order Fe > F2+ > F3+.
Question 34.
IE2 > IE1 for a given element — why?
Answer:
IE2 > IE1 for a given element
Reason : —
- IE1 means minimum amount of energy required to remove an electron from isolated neutral
gaseous atom. - IE2 means minimum amount of energy required to remove an electron from uni positive ion.
- In case of unipositive ion nuclear attraction increases on outer most electrons than in isolated gaseous atom. So more amount of energy needed to remove an electron from unipositive ion.
Hence IE2 > → IE1.
Question 35.
What is Ianthanide contraction? Give one of its consequences.
Answer:
Lanthanide contraction : Slow decrease in size of the atoms or ions among the lanthanides is known as lanthanide contraction.
Consequences due to lanthanide contraction:
- Due to lanthanide contraction, the crystall structure and other properties of the elements become very closely similar.
- Due to this, it becomes difficult to separate lanthanides from a mixture.
![]()
Question 36.
What is the atomic number of the element, having maximum number of unpaired 2p electrons ? To which group does it belong ?
Answer:
- The atomic number of the element having maximum no.of unpaired 2p electrons is 7′ (Z = 7)
- Element is nitrogen.
- Electronic configuration is 1s2 2s2 2p3 (3 unpaired 2p electrons)
Question 37.
Sodium is strongly metallic, while chlorine is strongly non-metallic – explain.
Answer:
Sodium is an alkali metal and it is present in group – I and it has the ability to lose the valency electron readily.
- It has high electropositive nature. So it has metallic nature.
- Chlorine is a halogen and it is placed in Group – 17 and it has the ability to gain the electron readily.
- It has high electronegative nature. So it has non metallic nature.
Question 38.
Why are zero group elements called noble gases or inert gases ?
Answer:
- Zero group elements has general outer electronic configuration ns2 np6 (except for He).
- These contains stable octet configuration. So these are stable and chemically inert. Hence these are called inert gases.
- These elements neither lose nor gain electrons. Hence these are called ‘noble gases’.
Question 39.
Select in each pair, the one having lower ionization energy and explain the reason,
a. I and I–
b. Br and K
c. Li and Li+
d. Ba and Sr
e. O and S
f. Be and B
g. N and O
Answer:
a) I– has lower ionisation energy than I because of increase of size \(\mathrm{I}^{\ominus}\) ion than ‘I’.
b) K has lower ionisation energy than ‘Br’ because of low electronegative value of K (0.8) than ‘Br’ (2.8).
c) ‘Li” has lower ionisation energy than Li+ because of large size of ‘Li’ than Li+.
d) ‘S’ has lower ionisation energy than ‘O’ because of large size of ‘S’ than ‘O’.
e) ‘B’ has lower ionisation energy than ‘Be’ because ‘Be’ has completely filled electronic configuration (1s2 2s2).
f) ‘O’ has lower ionisation energy than ‘N’ because ‘N’ has half filled electronic configuration (1s2 2s2 2p3).
Question 40.
IE1 of O < IE1 of N but IE2 of O > IE2 of N – Explain.
Answer:
- ‘N’ has half filled electronic configuration (1s2 2s2 2p3)
So IE1 of O < IE1 of ‘N’. - O+ ion has half filled electronic configuration (1s2 2s2 2p3)
So IE2 of O > IE1 of N.
Question 41.
Na+ has higher value of ionization energy than Ne, though both have same electronic configuration – Explain.
Answer:
Na+ has higher value of I.E. than Ne, though both have same electronic configuration.
Reason : –
- Both have electronic configuration 1s2 2s2 2p6
- In case of Na+ ion effective nuclear charge increases and size decreases than in ‘Ne’.
Question 42.
Which in each pair of elements has a more electronegative gain enthalpy ? Explain.
a. N or O
b. F or Cl
Answer:
a) Oxygen has high electronegative gain enthalpy than Nitrogen because ‘N’ has stable half filled electron configuration.
b) Chlorine (- 349 KJ /mole) has high electronegative gain enthalpy than Fluorine (- 328 KJ/mole) because ‘F’ has small size and more inter electronic repulsions.
Question 43.
Electron affinity of chlorine is more than that of fluorine – explain.
Answer:
Chlorine (- 349 KJ / mole) has high electronegative gain enthalpy than Fluorine (- 328 KJ/mole) because ‘F’ has small size and more inter electronic repulsions.
![]()
Question 44.
Which in each has higher electron affinity ?
a. F or Cl–
b. O or O-
c. Na+ or F
d. F or F–
Answer:
a) Fluorine has high electron affinity than Cl– ion because of inert gas configuration of Cl– ion.
b) Oxygen has high electron affinity than O– because O– has positive of 2nd electron affinity.
c) F has high electron affinity than Na+ because Na+ has inert gas configuration.
d) F has high electron affinity than F– because F– has inert gas configuration.
Question 45.
Arrange the following in order of increasing ionic radius :
a. Cl–, P-3, S-2, F–
b. Al+3, Mg+2, Na+, O-2, F–
c. Na+, Mg+2, K+
Answer:
a) The increasing order of ionic radius is F– < Cl– < S-2 < P-3
b) The increasing order of ionic radius is Al+3 < Mg+2 < Na+ < F– < O-2
c) The increasing order of ionic radius is Mg+2 < Na+ < K+
Question 46.
Mg+2 is smaller than O-2 in size, though both have same electronic configuration –
explain.
Answer:
Mg+2 and O-2 ions are iso electronic species.
In case of iso electronic species nuclear charge increases size of ion decreases. So Mg+2 has small size than O-2.
Question 47.
Among the elements B, Al, C and Si
a. Which has the highest first ionization enthalpy ?
b. Which has the most negative electron gain enthalpy ?
c. Which has the largest atomic radius ?
d. Which has the most metallic character ?
Answer:
a) Highest I.E. is possessed by the element carbon
b) Most negative gain enthalpy is for carbon (- 122 KJ/mole)
c) Large atomic radius is for Al (1.43 A)
d) Most metallic nature having element is ‘Al’.
Question 48.
Consider the elements N, P, O and S and arrange them in order of ;
a. Increasing first ionization enthalpy
b. Increasing negative electron gain enthalpy
c. Increasing non-metallic character
Answer:
a) Increasing first Ionisation energy order is S < P < O < N.
b) Increasing negative electron gain enthalpy order is N < P < O < S. .
c) Increasing non metallic nature order is P < N < S < O.
Question 49.
Arrange in given order :
a. Increasing EA :O, S and Se
b. Increasing IE1 : Na, K and Rb
c. Increasing radius : I–, I+ and I
d. Increasing electronegativity : F, Cl, Br, I
e. Increasing EA : F, Cl, Br, I
f. Increasing radius : Fe, Fe+2, Fe+3
Answer:
a) Increasing order of electron affinity is O < Se < S.
b) Increasing order of IE1 is Rb < K < Na.
c) Increasing order of radius is I+ < I < I–
d) Increasing order of electronegativity is I < Br < F < Cl
e) Increasing order of electron affinity is I < Br < F < Cl
f) Increasing order of radius is Fe+3 < Fe+2 < Fe
![]()
Question 50.
a. Name the element with highest ionization enthalpy
b. Name the family with highest value of ionization enthalpy.
c. Which element possesses highest electron affinity ?
d. Name unknown elements at the time of Mendeleef
e. Name any two typical elements.
Answer:
a) Highest I.E1 possessing element is ‘Helium’.
b) The family that possess highest values of I.E is Noble gases (or) inert gases.
c) Highest electron affinity element is ‘Chlorine’.
d) Unknown elements at the time of mendeleef are Germanium (Eka silicon). Scandium (Eka Alu-minium), Gallium (Eka Boron).
e) 3rd period elements are called typical elements
Eg : Al, Si, P, Na, Mg etc
Question 51.
a. Name any two bridge elements.
b. Name two pairs showing diagonal relationship.
c. Name two transition elements.
d. Name two rare earths.
e. Name two transuranic elements.
Answer:
a) Bridge elements 2nd period elements are called Bridge elements Eg : Be, B.
b) i) ‘Li’ diagonally relates with ‘Mg’,
ii) ‘Be diagonally relates with ‘Al’.
c) Scandium, Titanium, Vanadium, Chromium, etc., are examples of transition elements.
d) Lanthanides are called rare earths
Eg : Cerium (Ce), Prasodimium (Pr), Promethium (Pm).
e) Neptunium (Np), Californium (Cf), Fermium (Fm) are examples of transuranic elements.
Question 52.
On the basis of quantum numbers, justify that the 6th period of the periodic table should have 32 elements.
Answer:
6th period contains the subshells 6s, 4f, 5d, 6p
6s can accomodate two electrons (2 elements)
4f can accomodate 14 electrons (14 elements)
5d can accomodate 10 electrons (10 elements)
6p can accomodate 6 electrons (6 elements)
Total no.of electrons can accomodation 6th period are 2 + 14 + 10 + 6 = 32
∴ 6th period of periodic table contains 32 elements.
![]()
Question 53.
How did Moseley’s work on atomic numbers show that atomic number is a fundamental property better than atomic weight ?
Answer:
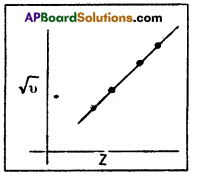
Mosley’s equation is
![]()
where v = frequency, Z = atomic number a, b = constants
A plot of \(\sqrt{v}\) against ‘Z’ gives a straight line.
However, no such relationship was obtained when the plot was drawn between frequency and the atomic mass. The atomic number of the elements, according to Mosley, stands for serial numbers of the elements in the periodic table. As the atomic number of the elements increase, the wavelengths of characteristic X – rays decrease. Mosley concluded that there is a fundamental quantity in an atom which increases in regular steps with increasing atomic number. The correlation between X – ray spectra and atomic number indicated that an element is characterized by its atomic number and not by atomic mass.
Question 54.
State modern periodic law. How many groups and periods are present in the long form of the periodic table ?
Answer:
Modern periodic law : – The physical and chemical properties of elements are periodic functions of their atomic numbers.
- In modern periodic table 7 periods and 18 groups are present.
Question 55.
Why f-block elements are placed below the main table ?
Answer:
In Lanthanides 4f – orbitals and in Actinides 5f – orbitals are filled. Since these elements have the same electronic configuration in the ultimate and penultimate shells they have similar properties. Hence they were placed at the bottom of the periodic table though they belongs to the sixth and seventh periods of IIIB groups.
Question 56.
Mention the number of elements present in each of the periods in the long form periodic table.
Answer:

Question 57.
Give the outer orbit general electronic configuration of
a. Noble gases
b. Representative elements
c. Transition elements
d. Inner transition elements
Answer:
Type of elements – General electronic configurations
a) Noble gases – ns2 np6 (except ‘He’ which has 1s2)
b) Representative elements – ns1-2 np0-5
c) Transition elements – (n – 1)d1-10ns1-2
d) Inner transition elements – (n – 2) f1-14(n – 1)d0, 1 ns2
Question 58.
Give any four characteristic properties of transition elements.
Answer:
Characteristic properties of elements:
a) They exhibit more than one oxidation state.
b) Most of the elements and their ions exhibit colour.
c) These elements and their compounds are good catalysis for various chemical processes.
d) They and their ions exhibit paramagnetic properties.
e) They form useful alloys.
Question 59.
What are rare earths and transuranic elements?
Answer:
- Lanthanides are rare earths. In these elements the differentiating electron enters into 4f – orbital.
- The elements present after Uranium are called Transurariic elements. All of these are radioactive and synthetic elements.
![]()
Question 60.
What is isoelectronic series? Name a series that will be isoelectronic with each of the following atoms or ions.
a. F–
b. Ar
c. He
d. Rb+
Answer:
The species containing same no.of electrons are called Iso electronic species and this series is called Isoelectronic series.
a) F– relating series
N-3, O-2, F–, Ne, Na+, Mg+2, Al+3
b) Ar relating series P-3, S-2, Cl–, Ar, K+, Ca+2
c) ‘He’ relating series H–, He, Li+, Be+2
d) Rb+ relating series
As-3, Se-2, Br–, Kr, Rb+, Sr+2
Question 61.
Explain why cation is smaller and anion is larger in radii than their parent atoms.
Answer:
- Cation means a positively charged species which is formed by an atom (or) element when an electron is lost.
M → M+ + \(\mathrm{e}^\Theta\) - Cation has high effective nuclear charge and decrease in size observed.
Hence cation has smaller radii. - Anion means a negatively charged species which is formed by an atom (or) element when an electron is gained.
M + e– → \(\mathrm{M}^{\Theta}\). - Anion has very low effective nuclear charge and increase in size observed.
Hence anion has larger radii.
Question 62.
Arrange the second period elements in the increasing order of their first ionization enthalpies. Explain why Be has higher IE1 than B.
Answer:
The increasing order of the I.E.s of 2nd period elements are as follows.

- Due to presence of incompletely filled p – orbitals in Boron, its IE value is less.
- Due to presence of completely filled s2 configuration in ‘Be’, it has higher IE value.
Question 63.
IE1 of Na is less than that of Mg but IE2 of Na is higher than that of Mg – explain.
Answer:
IE1 of Na is less than that of ‘Mg’
Reason :-
Na — has electronic configuration [Ne] 31
Mg — has electronic configuration [Ne] 3s2
Mg has completely filled configuration so Mg has more IE1 than Na.
IE2 of Na is higher than that of Mg
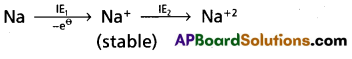
- Na+ has stable inert gas configuration so IE2 of Na is very high
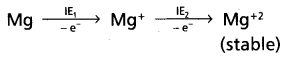
- By the lose of one electron from Mg+ ion forms Mg+2 ion which is more stable so low amount of energy is required.
∴ IE2 of Na is higher than Mg.
Question 64.
What are the various factors due to which the IE of the main group elements tends to decrease down a group ?
Answer:
The factors influencing on IE are
- Atomic radius
- Nuclear charge
- Screening effect
- Half filled, completely filled electronic configurations
- peretrating power
- In main group elements in a group IE decrease from top to bottom.
Reason: –
In groups from top to bottom size of elements increases hence IE values decreases.
Question 65.
The first ionization enthalpy values (in KJ mol-1) of group 13 elements are :

How do you explain this deviation from the general trend ?
Answer:
The given IE, values (in KJ / mole) of group 13 are as follow

- In genaral in a group IE values decrease down a group but from the above values we observe that there is no smooth decrease down the group.
- The decrease from B to Al is due to increase of size.
- The observed discontinuity in the IE values of Al and Ga, and between In and Tl are due to inability of d- and f – electrons, which have low screening effect to compensate the increase in nuclear charge.
![]()
Question 66.
Would you expect the second electron gain enthalpy of oxygen as positive, more negative or less negative than the first ? Justify.
Answer:
2nd gain enthalpy means energy released when an electron is added to uni negative ion.
O(g) + e– → \(\mathrm{O}_{(g)}^{-}\) + 141 KJ/mole
O(g) + e– → \(O_{(g)}^{-(2)}\) – 780 KJ/mole
- 2nd gain enthalpy of oxygen is positive because O– ion doesnot accept an electron readily and the entering electron have to over come the repulsive force.
Question 67.
What is the basic difference between the electron gain enthalpy and electropositivity?
Answer:
- Electron gain enthalpy means energy released when an electron is added to isolated neutral gaseous atom.
- The tendency to lose the electrons by an element is called electropositivity.
- Electron gain enthalpy is the measure of electronegativity.
- Electron gain enthalpy and electropositivity are inversely related.
Question 68.
Would you expect IE1 for two isotopes of the same element to be the same or different ? Justify.
Answer:
- Isotopes means existance of same elements with different mass no.s
- The isotope with higher mass no. have low I.E value than the normal isotope.
- This is due to the less nuclear attraction on valency electrons in case of heavier nuclide.
- But overall, the IE values of the isotopes are nearly same and the difference in IE values is negligible.
Question 69.
Increasing order of reactivity among group-1 elements is Li < Na < K < Rb < Cs, where as among group-17 elements it is F > Cl > Br > I- explain.
Answer:
a) Increasing order of reactivity among group -1 elements is Li < Na < K < Rb < Cs
Explanation: –
- Group -1 elements are Alkali metals.
- Group -1 elements have the tendency to lose the electrons
- Group -1 elements forms ionic bonds readily by losing electrons
- Group – 1 elements good reducing agents.
- Electro positive character increases from top to bottom this is due to increase of size.
b) Increasing order of reactivity among group -17 elements is F > Cl > Br > I
Explanation: –
- These are halogens (Group – 17)
- These have high electronegativity due to small size.
- These have the tendency to gain electrons.
- In a group from top to bottom electronegativity decrease, due to increase of size.
- These are oxidising agents and forms ionic bonds by gaining electrons.
Question 70.
Assign the position of the element having outer electronic configuration.
a. ns2np4 for n = 3
b. (n – 1)d2ns2 for n = 4
Answer:
a) ns2np4 for n = 3 .
- 3s23p4 → element is sulphur
- Sulphur belongs to VIA group (Group – 16) and 3rd period in periodic table,
b) (n – 1 )d2 ns2 for n = 4
3d2 4s2 → element is Titanium
- Titanium belongs to IVB group (Group – 4) and 4th period in the periodic table.
Question 71.
Predict the formulae of the stable binary compounds that would be formed by the combination of the following pairs of elements.
a. Li and O
b. Mg and N
c. Al and I
d. Si and O
e. p and Cl,
f. Element with atomic number 30 and Cl
Answer:
a) Stable binary compound formed between Li and O is Li2O (Lithiumoxide).
b) Stable binary compound formed between Mg and N is Mg3N2 (Magnesium nitride).
c) Stable binary compound formed between Al and I is AlI3 (Aluminium Iodide).
d) Stable binary compound formed between Si and I is SiO2 (Silicondioxide).
e) Stable binary compound formed between P and Cl is PCl3 and PCl5 (Phosphorous trichloride and phosphorous pentain chloride).
f) Stable binary compound formed between element with At. NO – 30 and Cl is ZnCl2 (Zinc chloride) [At. No – 30 – (Zn)].
Question 72.
Write a note on the variation of metallic nature in a group or in a period.
Answer:
Metals shows electropositive nature (i.e.,) loss of electrons and form positive ions.
Non – metals shows electronegative nature (i.e.,) gain of electrons and form negative ions.
Periodicity:
a) Down the group : Going down a group of the periodic table, the tendency to form positive ions, increases. That means there is an increase in metallic nature as the size of atom increases down the group.
b) Along a period : From left to right in a period, size of atom decreases. So there is decrease in metallic nature.
Question 73.
How does the covalent radius increase in group- 7 ?
Answer:
- Covalent radius increases in a group from top to bottom
- The increase of covalent radius in VIIA group elements as follows.

Question 74.
Which element of 3rd period has the highest IE1 ? Explain the variation of IE1 in this period.
Answer:
- In periods IE values increase from left to right
- Among 3rd period elements Argon (Ar) [Z = 18] possess highest IE,sub>1 value.
- IE1 values of 3rd period elements given below.
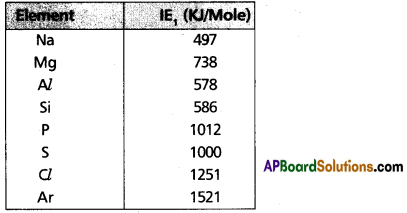
Exceptions:
- IE, of ‘Mg’ is higher than ‘Al’ because ‘Mg’ has completely filled ‘s’ subshells.
- IE, of ‘p’ is higher than ‘s’ because ‘p’ has half filled ‘p’ subshells.
Question 75.
What is valency of an element ? How does it vary with respect to hydrogen in the third period?
Answer:
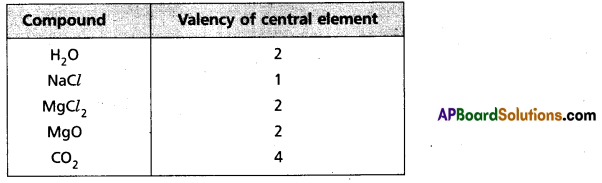
Valency : The combining capacity of an element with another element is called valency.
The number of hydrogen atoms (or) chlorine atoms (or) double the number of oxygen atoms, with which one atom of the element combine is also called valency.
∴ Valency = no. of hydrogens = no. of chlorine atoms
= 2 × no. of oxygen atoms present in the molecule.
e.g.:
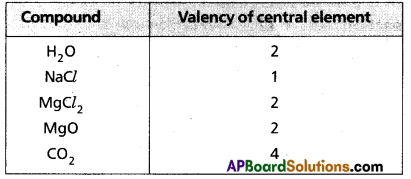
Periodicity of valency:
1) Each period starts with valency ‘1’ and ends in ‘0’.
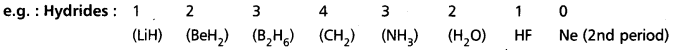
2) In a group valency is either equal to the group number (i.e., upto 4th group) or is equal to (8 – group number) (i.e., from 5th group onwards).
Significance :
Valency of an element is useful in writing the formulae of compounds.
![]()
Question 76.
What is diagonal relationship ? Give a pair of elements having diagonal relationship. Why do they show this relation ?
Answer:
On moving diagonally across the periodic table the elements show certain similarities. An element of group in 2nd period has similar properties with second element of the next higher group in the 3rd period. This type of resemblance is called diagonal relationship, e.g. : (Li, Mg); (Be, Al); (B, Si)
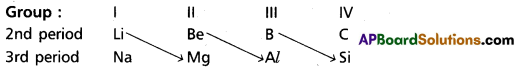
Diagonal relationship is due to similar sizes of atoms (or of ions) and similar electronegativities of the representative elements.
Diagonally similar elements posses the same polarizing power.
Polarizing power = \(\frac{\text { (ionic charge) }}{\text { (ionic radius) }^2}\)
Question 77.
What is lanthanide contraction ? What are its consequences ?
Answer:
Lanthanide contraction : Slow decrease in size of the atoms or ions among the lanthanides is known as lanthanide contraction.
Consequences due to lanthanide contraction:
- Due to lanthanide contraction, the crystall structure and other properties of the elements become very closely similar.
- Due to this, it becomes difficult to separate lanthanides from a mixture.
Question 78.
The first IP of lithium is 5.41 eV and electron affinity of Cl is – 3.61 eV. Calculate ΔH in kJ mol-1 for the reaction : Li(g) + Cl(g) → \(\mathrm{Li}_{(\mathrm{g})}{ }^{+}\) + \(\mathrm{Cl}{ }_{(g)}^{-}\)
Answer:
Given reaction is
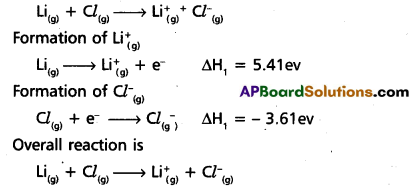
ΔH = ΔH1 + ΔH2
= 5.41 – 3.61
= 1.8 ev
= 1.8 × 9.65 × 104 J/mole
= 17.37 × 104 J/Mole
= 173.7 KJ/mole
Question 79.
How many Cl atoms can you ionize in the process Cl → Cl+ + e by the energy liberated for the process Cl + e → Cl– for one Avogadro number of atoms. Given IP = 13.0 eV and EA = 3.60 eV. Avogadro number = 6 × 1023
Answer:
Given
Cl(g) + e– → \(\mathrm{Cl}_{(\mathrm{g})}^{-}\) ΔH = – 3.6ev
1 – atom → Electron affinity = 3.6 ev
6.23 × 1023 atoms → ?
6.23 × 1023 × 3.6 = 21.6828 × 1023
∴ For one avagadro no.of Cl atoms electron affinity = 21.6828 × 1023 eV
Given 13 eV can ionise 1 atom of Cl
21.6828 × 1023 eV ionise -?
\(\frac{21.6828 \times 10^{23}}{13}\) = 1.667 × 1023 eV
Question 80.
The electron affinity of chlorine is 3.7 eV. How much energy in kcal is released when 2g of chlorine atoms is completely converted to Cl– ions in the gaseous state ? (1 e V = 23.06 kcal)
Answer:
Given electron affinity of Cl = 3.7 ev
Cl + e– → Cl– ΔH = – 3.7ev
35.5 gms of CZ contains 6.023 × 1023 atoms
2 gms of Cl contains ?
= \(\frac{2 \times 6.023 \times 10^{23}}{35.5}\) atoms
6.23 × 1023 atoms can liberate 3.7 eV
\(\frac{2 \times 6.023 \times 10^{23}}{35.5}\) atom can liberate?
= \(\frac{2 \times 6.023 \times 10^{23} \times 3.7}{35.5 \times 6.023 \times 10^{23}}\) = \(\frac{2 \times 3.7}{35.5}\) = \(\frac{7.4}{35.5}\)
= 0.2084 eV
= 0.2084 × 23.06 kcal/mole
= 4.81 k.cal/mole
Long Answer Questions
Question 1.
Discuss the classification of elements by Mendaleev’
Answer:
The periodic classification of elements based on “atomic weights” was done by “Lothar Meyer” and “Mendeleev” independently.
Mendeleev’s periodic law : “The physical and chemical properties of elements and their compounds are a periodic function of their atomic weights”.
Mendeleev arranged the 65 elements in a periodic table. He did not blindly follow the atomic weight but gave more importance to their chemical properties in arranging them in the table.
Explanation of the periodic law : When the elements are arranged in the increasing order of their atomic weights, elements with similar properties appear again and again, at regular intervals. This is called, periodicity of properties.
Mendeleev’s table : Mendeleev introduced a periodic table containing the known 65 elements. In this table, while arranging the elements, he gave importance only to their atomic weights, but also to their physical and chemical properties. This table was defective in some responses. Then he introduced another table, after rectifying the defects of that table. lt is called, “short form of periodic table”. He named the horizontal rows as ‘periods’ and the vertical columns, as ‘groups’. It has in all ‘9’ groups, I to VIII and a ‘O’ group. The first ‘7’ groups were divided into A and B sub groups. There are ‘7’ periods in the table. The VIII group contains three triods, namely, (Fe, Co, Ni), (Ru, Rh, Pd) and (Os, Ir, Pt).
Mendeleev’s observations :
- When the elements are arranged according to their atomic weights, they exhibit periodicity of properties.
- Elements with similar chemical properties have nearly equal atomic weights: Iron (55.85),. Cobalt (58.94) and Nickel (58.69).
- The group number corresponds to the valency of element in that group.
- Most widely distributed elements like H,C, O, N, Si, S etc., have relatively low atomic weights.
- The atomic weight of an element may be corrected if the atomic weights of the adjacent elements are known. The properties of an element are the average properties of the neighbouring elements.
- The atomic weights of beryllium. Indium, Uranium etc. were corrected, based on this observation.
Merits of Mendeleev’s table:
- Actually it formed the basis for the development of other modern periodic tables.
- Mendeleeffs left some vacant spaces in his periodic table, for the unknown elements. But the predicted the properties of those elements. Later on, when these elements were discovered, they exactly fitted into those vacant places having properties, predicted by Mendeleev.
Ex : Eka – boron (Scandium), EKa – Silicon (germanium)
EKa – aluminium (gallium) etc. - ‘O’ group elements were not known at the time of Mendeleeff. Later when they were discovered, they found a proper place in that table under ‘0’ group of elements. Similarly, the radioactive elements.
- In case of these pairs of elements Tellurium – Iodine Argon – Potassium and Cobalt – Nickel, there is a reversal of the trend. The first element has higher atomic weight than the second one. These are called anomalous pairs.
However, based on their atomic numbers, and chemical properties, this arrangement proves quite justified.
Draw-backs of Mendeleev’s periodic table :
- Dissimilar elements were placed in the same group.
Ex : The coinage mentals Cu, Ag and Au are placed along with the alkali metals K, Rb, Cs etc. in the I group. The only common property among them is that they are all univalent (Valency = 1). - The 14 rare earths having different atomic weights are kept in the same place.
- Hydrogen could not be given a proper place, as it resembles alkali metals and halogens in its properties.
![]()
Question 2.
From a study of properties of neighbouring elements, the properties of an unknown element can be predicted – Justify with an example.
Answer:
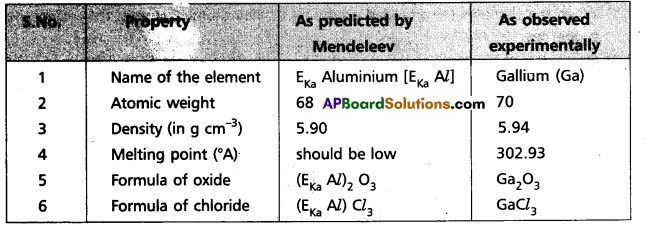
From a study of adjacent elements and their compounds, it is possible to predict the characteristics of certain elements. These predictions were found to be very accurate. These predicted properties helped the future scientists in the discovery of unknown elements, e.g. : EKa Aluminium (EKa Al) (now known as Gallium); EKa Silicon (EKa Si) (now known as Germanium); EKa Boron (EKa B) (now known as Scandium).
Illustration : Following table shows a comparison of the properties predicted by’Mendeleeff’ for the elements and those found experimentally after their discovery.
Question 3.
Discuss the construction of long form periodic table.
Answer:
The elements are arranged in the long form of the periodic table in the increasing order of atomic numbers. ‘Neils Bohr’ constructed the long form of the periodic table based on electronic configuration of elements.
The important features of the long form of the periodic table are :
It consists seven horizontal rows called periods and 18 vertical columns which are classified into 16 groups only.
Periods : Every period starts with an alkali metal and ends with an inert gas. The first period consists of two elements only (H, He) and is called very short period. Second period consists 8 elements (Li to Ne) and is called first short period. The third period consists (Na to Ar) 8 elements and is called second short period.
Fourth period contains 18 elements (K to Kr) and is called first long period. Fifth period is the second long period with 18 elements (Rb to Xe).
Sixth period is the longest period with 32 elements which starts with Cs and ends with Rn. This period includes 14 lanthanides.
Seventh period is an incomplete period with 20 radioactive elements.
Groups : There are 16 groups in the long form of the periodic table (in transition elements three vertical columns are fused and designated as VIII group). These groups are IA, IIA, NIB, IVB, VB, VIB, VIIB, VIII, IB, MB, IMA, IVA, VA, VIA, VIIA and zero group.
The elements of IA, IIA, IIIA, IVA, VA, VIA, VIIA groups are called representative elements or normal elements. Elements of IB, MB, NIB, IVB, VB, VIB, VIIB and VIII groups have their ultimate and penultimate shell incomplete. These are called transition elements. MB elements have (n – 1) d10 ns2 outermost electronic configuration.
Zero group elements have stable electronic configuration. These elements are called inert gases, noble gases. These elements have been grouped at the extreme right of the periodic table.
In this long periods have been expanded and short periods are broken to accommodate the transitional elements in the middle of the long period.
Lanthanides and actinides have been grouped separately and placed at the bottom of the periodic table.
Question 4.
Discuss the relation between the number of electrons filled into the sub energy levels of an orbit and the maximum number of elements present in a period.
Answer:
Elements have been accommodated in these periods according to the following scheme.
1st period : The first main energy shell (K – shell) is completed. As the maximum capacity of K- shell is of 2 electrons, it consists of only two elements, hydrogen (1s1)and helium (1s2).
2nd period : The second main energy shell is completed (i.e.,) 2s and 2p are completed. It includes eight elements from Li (2s1) to Ne (2s22p6).
3rd period : The 3s and 3p energy shells are completed. It includes also eight elements from Na(3s1) to Ar (3s2 3p6).
4th period : The 4s, 3d and 3p energy shells are completed. It includes 18 elements from K(4s1) to Kr (3d10 4s24p6). It includes two s – block elements, ten d – block elements and six p – block – elements.
5th period : The 5s, 4d and 5p energy shells are completed. It includes 18 elements from Rb (5s) to Xe (4d10 5s1 5p6). 1st also includes two s – block elements, ten d – block elements and six p – block elements.
6th period : The 6s, 4f, 5d and 6p energy shells are completed, (i.e.,) it includes 32 elements from Cs (6s1) to Rn (4f14 5d10 6s26p6). It consists of two s – block elements, ten d – block elements, six p – block elements and fourteen f – block elements.
As this period can accommodate only 18 elements in the table, 14 members of 4f – series are separately accommodated in a horizontal row below the periodic table.
7th period : This period at present consists 26 elements. The 7s, 5f and 6d are completed (i.e.,) twenty six elements. Seven of the elements from atomic numbers 106 to 112 have recently been reported.
![]()
Question 5.
Write an essay on s, p, d and f block elements .
Answer:
According to the electronic configuration of elements, the elements have been classified into four blocks. The basis for this classification is the entry of the differentiating electron into the subshell. They are classified into s, p, d and f blocks.
‘s’ block elements : If the differentiating electron enters into ‘s’ orbital, the elements belongs to ‘s’ block.
In every group there are two ‘s’ block elements. As an ‘s’ orbital can have a maximum of two electrons, ‘s’ block has two groups IA and IIA.
‘p‘ block elements : If the differentiating electron enters into ‘p’ orbital, the elements belongs to ‘p’ block.
‘p’ block contains six elements in each period. They are IIIA to VIIA and zero group elements. The electronic configuration of ‘p’ block elements varies from ns2np1 to ns2np6.
‘d‘ block elements : It the differentiating electron enters into (n – 1) d – orbitals the elements belongs to’d’ block. These elements are in between ‘s’ and ‘p’ blocks. These elements are also known as transition elements. In these elements n and (n – 1) shells are incompletely filled. The general electronic configuration of’d’ block elements is (n – 1) d1-10 ns1-2. This block consists of IIIB to VIIB, VIII, IB and IIB groups.
‘f’ block elements : If the differentiating electron enters into ‘f’ orbitals of antipenultimate shell (n – 2) of atoms of the elements belongs to ‘f block. They are in sixth and seventh periods in the form of two series with 14 elements each. They are known as lanthanides and actinides and are arranged at the bottom of the periodic table. The general electronic configuration is (n – 2)f1-14 (n – 1)d0 – 1 ns2.
In these shells the last three shells (ultimate, penultimate and anti penultimate) are incompletely filled. Lanthanides belongs to 4f series. It contains Ce to Lu. Actinides belong to 5f series. It contains Th to Lr.
Advantages of this kind of classification :
As a result of this classification of elements were placed in correct positions in the periodic table. It shows a gradual gradation in physical and chemical properties of elements. The metallic nature gradually decreases and non – metallic nature gradually increases from’s1′ block to ‘p1 block. This classification gave a special place for radioactive elements.
Question 6.
Relate the electronic configuration of elements and their properties in the Classification of elements.
Answer:
The chemical properties of all elements depends upon the electronic configuration. Upon the basis of complete and incomplete electron shells and chemical properties, the elements are classified into four types. (Type -1, Type – II, Type – III and Type – IV).
Type – I(inert gas elements): All the elements with an electronic configuration ns2 np6 including He belongs to this type, nth shell of those elements are completely filled.
The elements show chemical inertness due to completely filled shells and hence they have extra stability. Because of their stability, they are chemically inactive.
e.g. : He, Ne, Ar, Kr, Xe and Rn.
Type – II (Representative elements) : Except inert gases, the remaining elements of s and p – blocks are called representative elements. All the elements with an electronic configuration ns1 to ns2 np5 excluding. He comes under this type. These are atoms in which all except the outermost shells (nth) are complete. Elements of this type enter into chemical combination by loosing, gaining or sharing electrons to get stable inert gas configuration. Many of the metals, all non – metals and metalloids come under this type. Chemically these elements are reactive.
Type – III (Transition elements): All the elements with an electronic configuration (n – 1) d1 – 9 ns1or2 belongs to this type. Atoms in which the two outermost shells are incomplete. These elements show variable oxidation states, form complex ions and coloured ions. The electronic configuration of d – block elements is (n – 1) d1 – 10 ns1 – 2. Small size, high nuclear charge and unpaired’d’ orbitals impart characteristic properties to be transition elements.
Type- IV (Inner transition elements): All the elements with an electronic configuration (n – 2) f1 – 14 (n -1) d0, 1 ns2 belongs to this type. Atoms in which three outermost shells are incomplete. Lanthanides and Actinides belong to this type.
Question 7.
What is a periodic property ? How the following properties vary in a group and in a period ? Explain
a) Atomic radius
b) Electron gain enthalpy.
Answer:
Recurrence of similar properties of elements at definite regular intervals with increasing atomic number i.e., according to their electronic configurations is known as periodicity. Any property which is periodic in nature is called periodic property.
a) Atomic radius : The atomic radius decreases from left to right in a period. With an increase in the atomic number in a period the nuclear charge increases. As a result the effective nuclear charge over the outermost electrons increases, due to this the orbitals are pulled closer to the nucleus causing in a decrease in the atomic radius.
The atomic radius increases from top to the bottom in a group because – with an increase in the atomic number the electrons are added to new shells resulting an increase in the number of inner shells. Hence atomic radius increases from top to bottom in a group.
b) Electron gain enthalpy : Electron gain enthalpy increases in a period from left to right because the size of the atom decreases and the nature of the element changes from metallic to non – metallic nature when we move from left to right in a period.
Electron gain enthalpy decreases from top to bottom in a group because there is an increase in the atomic size. But the second element has greater electron gain enthalpy than the first element.
e.g. : Chlorine has more electron affinity value (- 348 kJ mol-1) than Fluorine (-333 kJ mol-1). It is because fluorine atom is smaller in size than chlorine atom. There is repulsion between the incoming electron and electrons already present in fluorine atom i.e., due to stronger inter electronic repulsions.
Question 8.
What is a periodic property ? How the following properties vary in a group and in a period ?
Explain
a. IE
b. EN
Answer:
a) IE : In groups and periods of the periodic table the ionization enthalpy values are periodically change depend upon the electronic configuration and size of elements.
In a group of elements ionization energy decreases from top to bottom because atomic radius increases.
In general, in a period the atomic size decreases. Because of this, the ionization energy increases across a period.
b) Electro negativity : Electronegativity increases from left to right in a period since the atomic: radii decreases and nuclear attraction increases.
In a group electronegativity decreases from top to bottom due to an increase in atomic radii and a decrease in nuclear attraction.
![]()
Question 9.
Write a note on
a. Atomic radius
b. Metallic radius
c. Covalent radius
Answer:
a) Crystal Radius (Atomic radius or Metallic radius): The term is used for metal atoms. A metallic crystal contains metal atoms in close packing. These metal atoms are considered spherical. They are supposed to touch each other in the crystal.
The crystal radius is half the internuclear distance between two adjacent atoms.
e.g.: The internuclear distance between two adjacent sodium atoms in a crystal of sodium metal is 3.72 A. So crystal radius of sodium is \(\frac{3.72}{2}\) = 1.86 A
For potassium it is 2.31 A.
b) Covalent radius : It is used generally for non – metals. Covalent radius is half the equilibrium distance between the nuclei of two atoms with a covalent bond.
Covalent radii of two atoms can be added to give internuclear distance between them.
e.g. : Covalent radius of H is 0.37A and for chlorine it is 0.99A. Hence internulear distance between H and Cl in HCl is 1.36A.
c) Vander Waals radius (Collision radius): The Vander Waals radius is half the equilibrium distance between the nuclei of two atoms bound by Vander Waals forces.
It is used for molecular substances in solid state and for inert gases.
e.g. : The Vander Waals radius for hydrogen is 1.2 A and that of chlorine is 1.80 A.
The Vander Waals radius of an atom is 40% larger than its covalent radius.
Question 10.
Define IE1 and IE2. Why is IE2 > IE1 for a given atom? Discuss the factors that effect IE of an element. (A.P. Mar. ’16)(T.S. Mar. ’13)
Answer:
1) Ionization energy is the amount of energy required to remove the most loosely held electron from isolated a neutral gaseous atom to convert it into gaseous ion. It is also known as first ionization energy because it is the energy required to remove the first electron from the atom.
It is denoted as I1, and is expressed in electron volts per atom, kilo calories (or) kilo joules per mole.
M(g) + I1 → \(M_{(g)^{+}}\) + e–
I1 is first ionization potential.
2) The energy required to remove another electron from the unipositive ion is called the second ionization energy. It is denoted as I2.
\(M_{(g)}^{+}\) + I2 → \(\mathrm{M}_{(\mathrm{g})}{ }^{2+}\) + e–
3) The second ionization potential is greater than the first ionization potential. On removing an electron from an atom, the unipositive ion formed will have more effective nuclear charge than the number of electrons. As a result the effective nuclear charge increases over the outermost electrons. Hence more energy is required to remove the second electron. This shows that the second ionization potential is greater than the first ionization potential.
For sodium, I1 is 5.1 eV and I2 is 47.3 eV.
I1 < I2 < I3 ….. In
Factors affecting ionization potential:
1. Atomic radius : As the size of the atom increases the distance between the nucleus and the outermost electrons increases. So the effective nuclear charge on the outermost electrons decreases. In such a case the energy required to remove the electrons also decreases. This shows that with an increase in atomic radius the ionization energy decreases.
2. Nuclear charge : As the positive charge of the nucleus increases its attraction increases over the electrons. So it becomes more difficult to remove the electrons. This shows that the ionization energy increases as the nuclear charge increases.
3. Screening effect or shielding effect: In multielectron atoms, valence electrons are attracted by the nucleus as well as repelled by electrons of inner shells. The electrons present in the inner shells screen the electrons present in the outermost orbit from the nucleus. As the number of electrons in the inner orbits increases, the screening effect increases. This reduces the effective nuclear charge over the outermost electrons. It is called screening or shielding effect. With the increase of screening effect the ionization potential decreases. Screening efficiency of the orbitals falls off in the order s > p > d > f.
(Magnitude of screening effect) ∝ \(\frac{1}{(\text { Ionization enthalpy) }}\)
TREND IN A GROUP : The ionisation potential decreases in a group, gradually from top to bottom as the size of the elements increases down a group.
TREND IN A PERIOD : In a period from left to right I.P. value increases as the size of the elements decreases along the period.
Question 11.
How do the following properties change in group-1 and in the third period ? Explain with example.
a. Atomic radius
b. IE
c. EA
d. Nature of oxides
Answer:
a) Atomic radius
- In Group – 1 atomic radius from Li to Cs increases
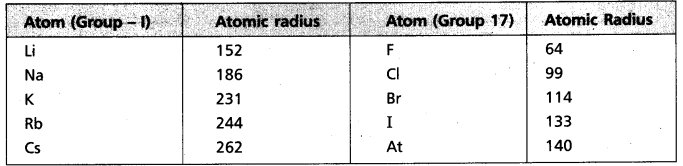
→ In 3rd period from Na to Cl atomic radius decreases.
b) Ionization energy : In a group ionization energy values decreases with an increase in the size of the atom. In IA group Li is the element with highest ionization potential and Cs is the element with lowest ionization potential.
In third period the ionization potential increases from Na to Mg and then decreases at Al and increases upto P and decreases in case of S and then increases upto argon, i.e., Al has a lower value than Mg and S has a lower value than P, due to stable electronic configurations of Mg and P Among these elements argon has highest ionization potential.
c) Electron affinity :
- In 3rd period E.A > from ‘Si’ to ‘P’ decreases and P to Cl increases.
‘Mg’ and ‘Ar’ has positive values. - In 1st group from Li to Cs electron affinity decreases due to increase of size.
d) Nature of oxides of elements : All IA group elements are alkali metals. Their oxides are basic in nature. They dissolve in water to give basic solutions which changes fed litmus blue.
e.g. : Na2O, CaO , MgO etc.
Na2O + H2O → 2 NaOH
CaO + H2O → Ca(OH)2
MgO + H2O → Mg (OH)2
The basic nature of these oxides increases from top to bottom in the group. In a period basic properties decreases and acidic properties increases.
![]()
Question 12.
Define electron gain enthalpy. How it varies in a group and in a period? Why is the electron gain enthalpy of O or F is less negative than that of the succeeding element in the group ?
Answer:
- Electron affinity is the amount of energy released when an electron is added to a neutral gaseous atom in its ground state. It is known as first Electron affinity EA,sub>1. It has -ve value.
X(g) + e → \(\mathrm{X}_{(\mathrm{g})}{ }^{-}\) + EA1 - Energy can be absorbed when an other electron is added to uni negative ion. It is because to overcome the repulsion between negative ion and electron. Hence second electron affinity, EA2 has +ve value.
\(X_{(g)}{ }^{-}\) + e → \(X_{(g)}^{2-}\) + EA2 - It can be indirectly determined from Born —Haber cycle. Its units are kJ/mole.
- Trend in a group : Generally EA decreases down the group. In a group the second element has higher EA value than the first member.
e.g. : EA of Cl is more than that of F
Fluorine possess lower EA value than chlorine. It is due to inner electron repulsions in Fluorine. - Trend in a period : Generally EA value increases across a period, but some irregularities can be observed.
a) I A group elements possess low EA values than the corresponding III A elements, e.g. : Be has EA value zero, it is due to completely filled 2s2 orbital.
b) VA elements have low EA values than that of VI A elements.
e.g. : Due to presence of half filled p – orbitals [2s2 2p3], nitrogen has lower EA value than that of oxygen.
c) For inert gases EA value is zero.
d) The element with the highest EA value is chlorine.
- The electron gain enthalpy of O and F is less negative than the succeeding element in the group because. These have small size and inter electronic repulsions are high in these elements
O → 141 KJ / mole and S → 200 kJ/Mole
F → 328 KJ / mole and Cl → 349 kJ/Mole
Question 13.
a. What is electronegativity ?
b. How does it vary in a group and in a period ?
Answer:
a) Electronegativity : ‘The tendency of the atom of an element to attract the shared electron pair(s) more towards itself in a hetronuclear diatomic molecule or in a polar covalent bond.” Measuring electronegativity Pauling scale : Pauling scale is based on the values of bond energy. The bond energy of a compound A – B is the average of bond energies of A – A and B – B molecules.
EA – B = \(\frac{1}{2}\left(E_{A-A}+E_{B-B}\right)\)
But the experimental value of EA – B is found to exceed the theoretical value. The difference is ∆.
∴ ∆ = E’A – B – EA – B
∆ indicates the polarity of the covalent bond. It is measured in k. cal. mol-1.
Pauling gave the relation XA – XB = 0.208 × \(\sqrt{\Delta}\)
In S.l. units XA – XB = 0.1017 \(\sqrt{\Delta}\) where ∆ is measured in kJ/mole
XA and XB are the electronegativities of A and B. Pauling arbitrarily fixed 2.1 as the electronegativity value of Flydrogen and calculated the electronegativities of other elements. On Pauling scale Fluorine has the highest EN value 4.0.
From the values of the electronegativity of elements, the nature of the chemical bond formed can be understood. If two bonded atoms differ by 1.70 or more ion their EN values, the bond between them would be either 50% or more than 50% ionic in nature. Similarly if the difference in the EN values of the atoms is less than 1.70, the bond formed is more than 50% covalent in nature.
b) Variation in a group and period : Electronegativity increases from left to right in a period since the atomic: radii decreases and nuclear attraction increases. In a group electronegativity decreases from top to bottom due to an increase in atomic radii and a decrease in nuclear attraction.
Question 14.
Explain the following
a. Valency
b. Diagonal relation
c. Variation of nature of oxides in the Group -1
Answer:
a) Valency : The combining capacity of an element with another element is called valency. The number of hydrogen atoms (or) chlorine atoms (or) double the number of oxygen atoms, with which one atom of the element combine is also called valency.
∴ Valency = no. of hydrogens
= no. of chlorine atoms
= 2 × no. of oxygen atoms present in the molecule
Periodicity of valency :
1) Each period starts with valency’11 and ends in ‘O’.
e.g.:
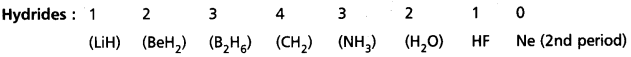
2) In a group valency is either equal to the group number (i.e., upto 4th group) or is equal to (8 – group number) (i.e., from 5th group onwards).
Significance :
Valency of an element is useful in writing the formulae of compounds.
b) Diagonal relation : On moving diagonally across the periodic table the elements show certain similarities. An element of group in 2nd period has similar properties with second element of the next higher group in the 3rd period. This type of resemblance is called diagonal relationship,
e.g. : (Li, Mg); (Be, Al); (B, Si)
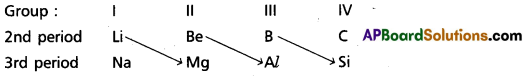
c) Nature of oxides of elements : All IA group elements are alkali metals. Their oxides are basic in nature. They dissolve in water to give basic solutions which changes red litmus blue.
e.g. : Na2O, CaO , MgO etc.
CaO + H2O → Ca(OH)2
MgO + H2O → Mg (OH)2
The basic nature of these oxides increases from top to bottom in the group. In a period basic properties decreases and acidic properties increases.
Solved Problems
Question 1.
What would be the IUPAC name and symbol for the element with atomic number 120?
Solution:
The roots for 1, 2 and 0 are un, bi and nil, respectively. Hence, the symbol and the name respectively are Ubn and unbinilium.
Question 2.
How would you justify the presence of 18 elements in the 5t” period of the Periodic Table ?
Solution:
When n = 5, l = 0, 1, 2, 3. The order in which the energy of the available orbitals 4d, 5s and 5p increases is 5s < 4d < 5p. The total number of orbitals available are 9. The maximum number of electrons that can be accommodated is 18; and therefore 18 elements are there in the 5th period.
![]()
Question 3.
The elements Z = 117 and 120 have not yet been discovered. In which family / group would you place these elements and also give the electronic configuration in each case.
Solution:
we see from that element with Z = 117, would belong to the halogen family (Group 17) and the electronic configuration would be [Rn] 5f146d107s27p5. The element with Z = 120, will be placed in Group 2 (alkaline earth metals), and will have the electronic configuration [Uuo]8s2
Question 4.
Considering the atomic number and position in the periodic table, arrange the following elements in the increasing order of metallic character: Si, Be, Mg, Na, P.
Solution:
Metallic character increases down a group and decreases along a period as we move from left to right. Hence the order of increasing metallic character is : P < Si < Be < Mg < Na.
Question 5.
Which of the following species will have the largest and the smallest size ?
Mg, Mg2+, Al, Al3+.
Solution:
Atomic radii decrease across a period. Cations are smaller than their parent atoms. Among isoelectronic species, the one with the larger positive nuclear charge will have a smaller radius.
Hence the largest species is Mg; the smallest one is Al3+.
Question 6.
The first ionization enthalpy (∆iH) values of the third period elements, Na, Mg and Si are respectively 496, 737 and 786 kJ mol-1. Predict whether the first ∆iH value for Al will be more close to 575 or 760 kJ mol-1 ? Justify your answer.
Solution:
It will be more close to 575 kJ mol-1. The value for Al should be lower than that of Mg because of effective shielding of 3p electrons from the nucleus by 3s-electrons.
Question 7.
Which of the following will have the most negative electron gain enthalpy and which the least negative ? P, S, Cl, F. Explain your answer.
Solution:
Electron gain enthalpy generally becomes more negative across a period as we move from left to right. Within a group, electron gain enthalpy becomes less negative down a group. However, adding an electron to the 2p-orbital leads to greater repulsion than adding an electron to the larger 3p-orbital. Hence the element with most negative electron gain enthalpy is chlorine; the one with the least negative electron gain enthalpy is phosphorous.
Question 8.
Using the Periodic Table, predict the formulas of compounds which might be formed by the following pairs of elements;
(a) silicon and bromine
(b) aluminium and sulphur.
Solution:
(a) Silicon is group 14 element with a valence of 4; bromine belongs to the halogen family with a valency of 1. Hence the formula of the compound formed would be SiBr4.
(b) Aluminium belongs to group 13 with a valence of 3; sulphur belongs to group 16 elements with a valence of 2. Hence, the formula of the compound formed would be Al2S2.
![]()
Question 9.
Are the oxidation state and covalency of Al in [AICI(H2O)5]2+ same ?
Solution:
No. The oxidation state of Al is +3 and the covalency is 6.
Question 10.
Show by a chemical reaction with water that Na2O is a basic oxide and Cl2O7 is an acidic oxide.
Solution:
Na2O with water forms a strong base Whereas Cl2O7 forms strong acid.
Na2O + H2O → 2NaOH
Cl2O7 + H2O → 2HClO4
Their basic or acidic nature can be qualitatively tested with litmus paper.