Students get through AP Inter 2nd Year Chemistry Important Questions Lesson 12(a) Alcohols, Phenols, and Ethers which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions Lesson 12(a) Alcohols, Phenols, and Ethers
Very Short Answer Questions
Question 1.
Explain why propanol has higher boiling point than that hydrocarbon-butane.
Answer:
Propanol has higher boiling point (391K) than that hydrocarbon butane (309K).
Reason : In propanol strong intermolecular hydrogen bonding is present between the molecules. But in case of butane weak vander waal’s force of attractions are present.
Question 2.
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer:
Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses.
Explanation:
- Alcohols and water are both polar solvents. Alcohol is dissolves in water, due to formation of hydrogen bonding with water molecules.
- Hydro carbons are non polar and these donot form hydrogen bonds with water molecules. So alcohols are soluble in water where as hydrocarbons are not soluble in water.
![]()
Question 3.
Name the reagents used in the following reactions.
- Oxidation of primary alcohol to carboxylic acid
- Oxidation of primary alcohol to aldehyde.
Answer:
- The reagents used for the oxidation of 1° – alcohols to carboxylic acid are acidified K2Cr2O7 (or) Acidic/alkaline KMnO4 (or) Neutral KMnO4.
- The reagents used for the oxidation of 1°- alcohols to aldehyde are pyridine chloro chromate (PCC) in CH2Cl2.
Question 4.
Write any one method for the preparation of ethyl alcohol.
Answer:
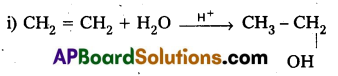
From Alkenes : Alcohols can be prepared from alkenes by hydration or hydroboration oxidation.
Question 5.
What is Williamsons synthesis ? Give example. (IPE – 2016 (AP) (TS), 2015 (AP))
Answer:
Williamsons synthesis : Reaction of alkyl halide with sodium alkoxide to give ether is called Williamson’s synthesis.
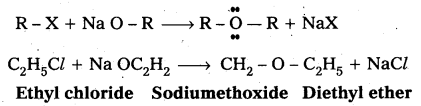
Question 6.
Write the equations for the following reactions.
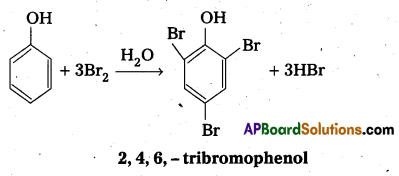
- Bromination of phenol to 2, 4, 6-tribromophenol
- Benzyl alcohol to benzoic acid.
Answer:
- Bromination of phenol to 2, 4, 6 tribromophenol.
- Benzyl alcohol to benzoic acid converted as follows.
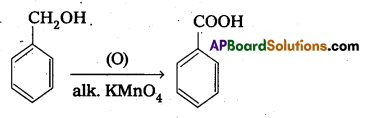
![]()
Question 7.
Give the reagents used for the preparation of phenol from chlorobenzene.
Answer:
Phenol is prepared from chlorobenzene as follows. Reagents required are
- NaOH, 623K, 300 atm,
- HCl.
Chemical reaction:
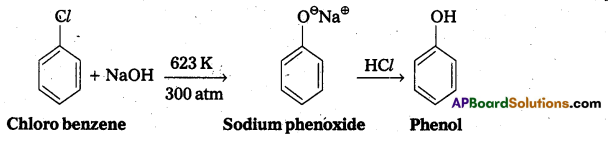
Question 8.
what is Esterification ? Give equation.
Answer:
Esterification : Alcohols react with carboxylic acids or acid halides or acid anhydrides to form esters.
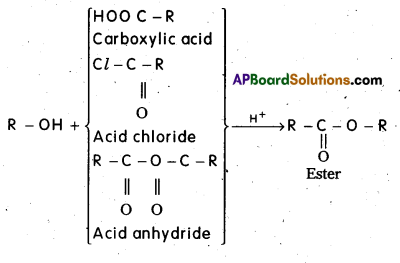
Question 9.
What is Dehydration ? Give equation.
Answer:
Dehydration : Alcohols undergo dehydration in the presence of dehydrating agents like cone H2SO4, (or) H3PO4 etc and form alkenes. The relative ease of dehydration of alcohols follows the following order. Tertiary alcohol > Secondary alcohol > primary alcohol.
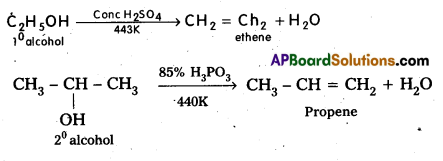
Question 10.
What is Reimer Tiemann reaction ? Give equation.
Answer:
Reimer-Tiemann reaction : Phenol reacts with chloroform in presence of NaOH to form salicylaldehyde (O-Hydroxy benzaldehyde). This reaction is known as Reimer-Tiemann reaction.
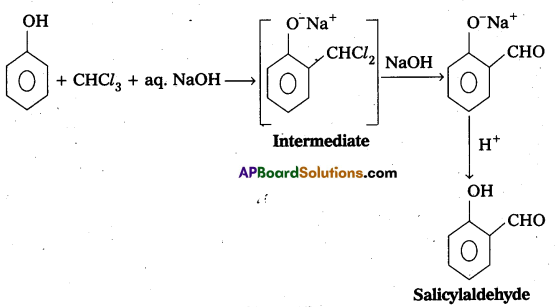
Question 11.
What is Kolbe’s reaction ? Give equation.
Answer:
Kolbe’s reaction : Phenol reacts with sodium hydroxide to form sodium phenoxide. This undergoes electrophillic substitution with CO2 to form salicylic acid.
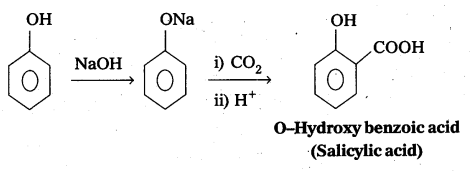
Question 12.
Write the mechanism of the reaction of HI with methoxymethane.
Answer:
Case – I : When methoxy methane reacts with cold.dil. HI then methyl alcohol and methyl iodide are formed.
Mechanisms:
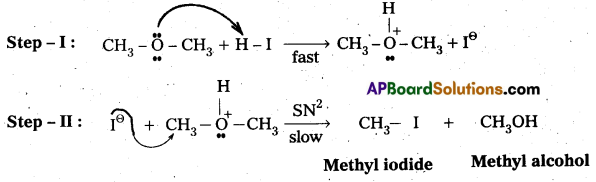
Case-II: When methoxy methane reacts with hot.conc.HI then only methyl iodide is formed.
Mechanisms:
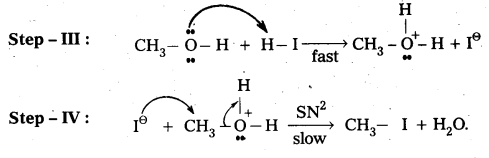
![]()
Question 13.
Identify the reactant needed to form t-butylalcohol from acetone.
Answer:
When acetone reacts with methyl magnesium bromide followed by the hydrolysis forms t-butylalcohol.
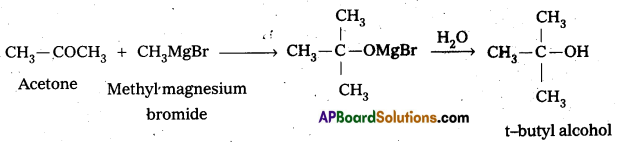
Question 14.
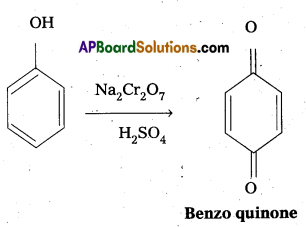
Write the Oxidation reaction of phenol.
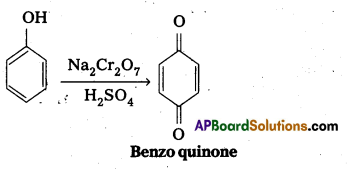
Answer:
Phenol undergo oxidation with chromicacid and forms a conjugated diketone known as benzoquinone.
Short Answer Questions
Question 1.
Give the equations for the preparation of phenol from Cumene. (TS Mar. ’17) (Mar. ’14)
Answer:
Phenol is prepared from Cumene as follows.
- Oxidation of Cumene to Cumene hydroperoxide.
- Cumene hydroperoxide on acidic hydrolysis to form phenol.
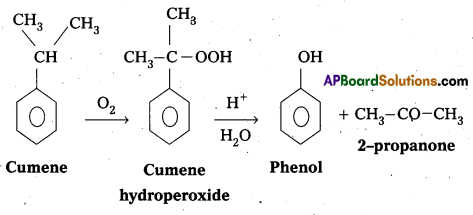
Question 2.
Explain the acidic nature of phenols and compare with that of alcohols. (AP Mar. ’17)Board Model Paper
Answer:
The reaction of phenol with sodium metal and with aq.NaOH indicates the acidic nature of phenol.
i) Phenol reacts with sodium metal to form sodium phenoxide.
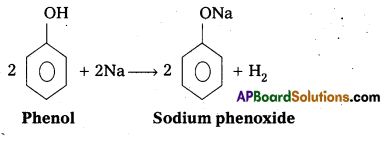
ii) Phenol reacts with aq.NaOH and forms sodium phenoxide
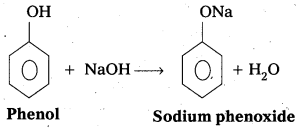
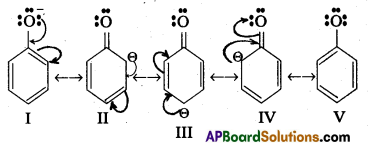
- In phenol hydroxyl group is attached to the Sp2 hydridised carbon of benzene ring which acts as electron with drawing group. The formed phenoxide ion from phenol is more stabilised due to delocalisation of negative charge.
Comparison of acidic character of Phenol and Ethanol:
- The reaction of phenol with aq. NaOH indicates that phenols are stronger acids than alcohols.
- The hydroxyl group attached to an aromatic ring is more acidic than in hydroxyl group is attached to an alkyl group.
- Phenol forms stable phenoxide ion stabilised by resonance but ethoxide ion is not.
Question 3.
Write the products formed by the reduction and oxidation of phenol.
Answer:
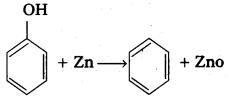
a) Reduction of phenol: Phenol undergo reduction in presence of zinc dust to form benzene.
b) Oxidation of phenol : Phenol undergo oxidation with chromic acid and forms a conjugated diketone known as benzoquinone.
Question 4.
Explain why in anisole electrophilic substitution takes place at ortho and para positions and not at meta position.
Answer:
Anisole is an aryl alkyl ether. In anisole the group -OCH3 influences +R effect. This increases the electron density in the benzene ring and it leads to the activation of benzene ring towards electrophilic substitution reactions.
In Anisole eletron density is more at O—and P—positions but not at M—position. So O—and P-products are mainly formed during electrophillic substitution reactions.
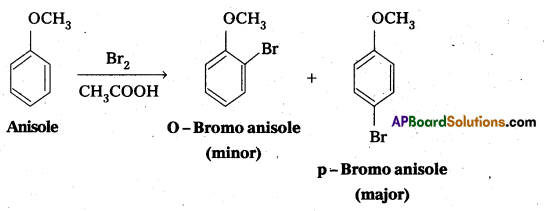
![]()
Question 5.
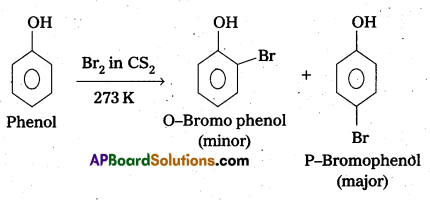
Explain whý phenol with bromine water forms 2,4, 6-tribromophenol while on reaction with bromine in CS2 at low temperatures forms para—bromophenol as the major product.
Answer:
a) Phenol undergoes Bromination in presence of CS2 to form p—bromophenol as major product.
b) Phenol undergoes bromination in aqueous medium form 2,4,6 -tribromo phenol (white ppt).
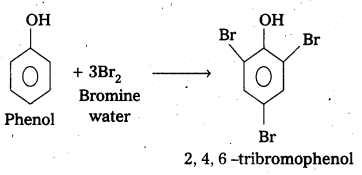
Explanation : In bromination of phenol, the polarisation of Br2 molecule takes place even in the absence of Lewis acid. This is due to the highly activating effect of -OH group attached to the benzene ring.
Question 6.
Explain the acidic nature of phenol.
Answer:
The reaction of phenol with sodium metal and with aq.NaOH indicates the acidic nature of phenol.
i) Phenol reacts with sodium metal to form sodium phenoxide.
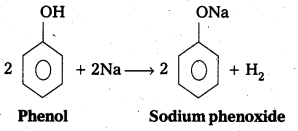
ii) Phenol reacts with aq.NaOH and forms sodium phenoxide.
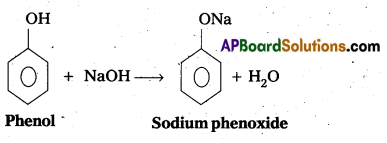
- In phenol hydroxyl group is attached to the Sp2 hydridised carbon of benzene ring which acts as electron with drawing group. The fõrmed phenoxide ion from phenol is more stabilised due to delocalisation of negative charge.
Question 7.
Explain the electrophilic substitution reaction of Anisole.
Answer:
Electrophilic substitution in Anisole:
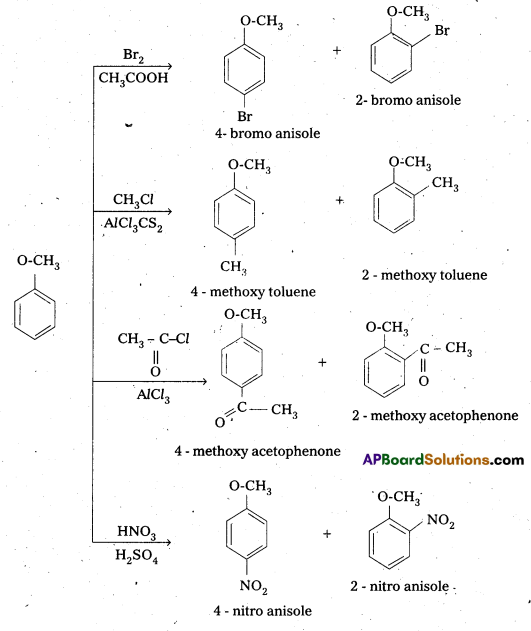
In all the above reactions p – isomer is the major product.
Question 8.
Write equations of the below given reactions:
i) Alkylatlon of anisole
ii) Nitration of anisole
iii) Friedel—Crafts acetylation of anisole
Answer:
i) Friedel crafts alkylation of anisole:
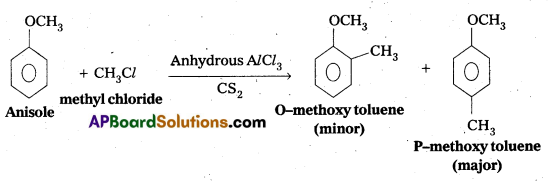
ii) Nitration of anisole:
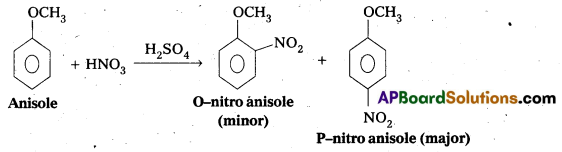
iii) Friedel – Crafts acetylation of anisole
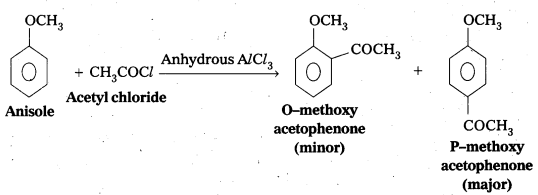
Question 9.
Illustrate hydroboration-oxidation reaction with a suitable example.
Answer:
When alkenes undergo addition reaction with diborane to form tri alkyl boranes. These followed by the oxidation by alkaline H2O2 to form alcohols. This reaction is called as hydroboration-oxidation reaction.
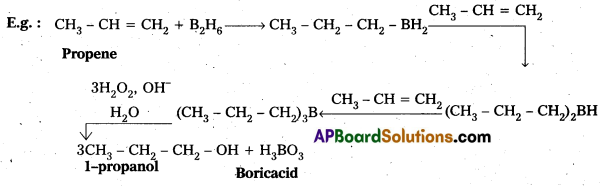
Question 10.
Write any two methods for the preparation of phenol. (IPE 2014)
Answer:
Preparation of Phenol : Phenol can be prepared from halobenzene, benzene diazonium chloride and cumene etc.
i) From halobenzene
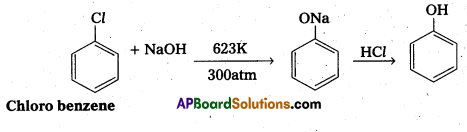
ii) from Benzene diazonium chloride
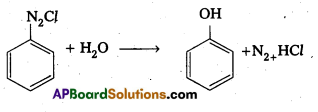
![]()
Question 11.
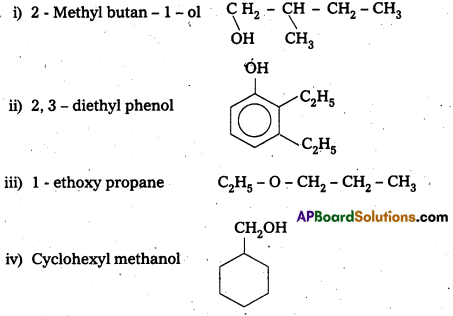
Write the structures of the following compounds.
i) 2 Methyl butan -1 – o1
ii) 2, 3 – diethyl phenol
iii) 1 – ethoxy propane
iv) Cyclohexyl methanol
Answer: