Students get through AP Inter 2nd Year Chemistry Important Questions Lesson 3(a) Electro Chemistry which are most likely to be asked in the exam.
AP Inter 2nd Year Chemistry Important Questions Lesson 3(a) Electro Chemistry
Very Short Answer Questions
Question 1.
What is a galvanic cell or a voltaic cell ? Give one example.
Answer:
Galvanic cell: A device which converts chemical energy into electrical energy by the use of spontaneous redox reaction is called Galvanic cell (or) voltaic cell.
Eg.: Daniell cell.
Question 2.
What is standard hydrogen electrode ?
Answer:
The electrode whose potential is known as standard electrode (or) standard hydrogen electrode.
To determine the potential of a single electrode experimentally it combine with standard hydrogen electrode and the EMF of cell so constructed is measured with potentiometer.
![]()
Question 3.
What is Nernst equation ? Write the equation for an electrode with electrode reaction
Mn+ (aq) + ne– \(\rightleftharpoons\) M(s).
Answer:
The electrode potential at any concentration measured with respect to standard hydrogen electrode is represented by Nernst equation.
Nernst equation is
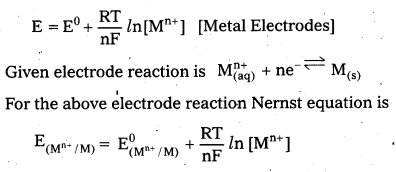
Here, E(Mn+/M) = Electrode potential
E0(Mn+/M) = Standard electrode potential
R = gas constant = 8.3 14 J/k.mole
F = Faraday = 96487 c/mole
T = temperature .
[Mn+] = concentration of species Mn+
Question 4.
How is E0 cell related mathematically to the equilibrium constant Kc of the cell reaction?
Answer:
Relation between E0 cell and equilibrium constant Kc of the cell reaction
\(E_{\text {cell }}^0\) = \(\frac{2.303 \mathrm{RT}}{\mathrm{nF}} \log \mathrm{K}_{\mathrm{c}}\)
n = number of electrons involved
F = Faraday = 96500 C mol-1
T = Temperature
R = gas constant
Question 5.
How is Gibbs energy (G) related to the cell emf (E) mathematically ?
Answer:
Relation between Gibb’s energy (G) and emf (E) mathematically
ΔG° = – nFE(cell)
ΔG = change in Gibb’s energy
n = number of electrons involved
F = Faraday = 96500 C mol-1
![]()
Question 6.
Define conductivity of a material. Give its SI units.
Answer:
The reciprocal of specific resistance (or) resistivity is called conductivity. It is represented by (K)
(Or)
The conductance of one unit cube of a conductor is also called conductivity.
SI units : ohm-1 m-1 (or) Sm-1 S = Siemen
Question 7.
What is cell constant of a conductivity cell ?
Answer:
Cell constant:
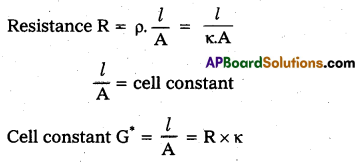
The cell constant of a conductivity cell is the product of resistance and specific conductance.
Question 8.
State Faraday’s second law of electrolysis.
Answer:
The amounts of different substances liberated when the same quantity of electricity is passed through the electrolytic solution are proportional to their chemical equivalent weights.
m ∝ E
Question 9.
State Kohlrausch’s law of independent migration of ions.
Answer:
Kohlrausch’s law of independent migration of ions : The limiting molar conductivity of the electrolytes can be represented as the sum of the individual contributions of the anion and the cation of the electrolytes.
\(\lambda_{m(A B)}^0\) = \(\lambda_{\mathrm{A}^{+}}^0\) + \(\lambda_{\mathrm{B}^{-}}^0\)
\(\lambda_m^0\) = Limiting molar conductivity
\(\lambda_{\mathrm{A}^{+}}^0\) = Limiting molar conductivity of cation ‘
\(\lambda_{\mathrm{B}^{-}}^0\) = Limiting molar conductivity of anion
Question 10.
State Faraday’s first law of electrolysis.
Answer:
The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of current passing through the electrolyte.
(Or)
The mass of the substance deposited at an electrode during the electrolysis of electrolyte is directly proportional to quantity of electricity passed through it.
m ∝ Q; m ∝ c × t
m = ect; m = \(\frac{\text { Ect }}{96,500}\)
e = electrochemical equivalent
t = time in seconds
c = Current in amperes
E = Chemical equivalent
Question 11.
What is a primary battery? Give one example. (AP Mar. ’17)
Answer:
The batteries which after their use over a period of time, becomes dead and the cell reaction is completed and this cannot be reused again are called primary batteries.
Eg: Leclanche cell, dry cell.
![]()
Question 12.
Give one example for a secondary battery. Give the cell reaction.
Answer:
Lead storage battery is an example of secondary battery.
The cell reactions when the battery is in use are
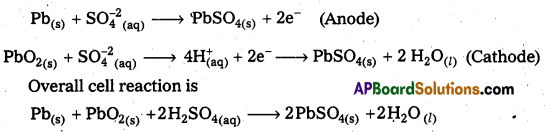
Question 13.
What is metallic corrosion? Give one example. (TS Mar. 2017, IPE ‘15 (AP))
Answer:
Metallic corrosion : The natural tendency of conversion of a metal into its mineral compound form on interaction with the environment is known as metallic corrosion.
Eg:
- Iron converts itself into its oxide.
[Rusting] (Fe2O3) - Silver converts itself into it’s sulphate
[tarnishing] [Ag2S]
Question 14.
Define electrochemical equivalènt (e.c.e).
Answer:
The weight of the substance deposited or liberated when one ampere of current is passed for 1 second (1 coloumb) is called electrochemical equivalent (e.c.e).
Question 15.
A solution of CuSO4 is electrolysed for 10 minutes with a current of 1.5 amperes. What is the mass of copper deposited at the cathode? (IPE 2015 (AP), 14)
Solution:
t =600 s charge = current × time = 1.5A × 600 s = 900 C
According to the reaction:
![]()
We require 2F or 2 × 96487 C to deposit 1 mol or 63 g of Cu.
Question 16.
Can you store copper sulphate solutions in a zinc pot ?
Solution:
No, zinc pot cannot store copper sulphate solutions because the standard electrode potential (E0) value of zinc is less than that of copper. So, zinc is stronger reducing agent than copper.
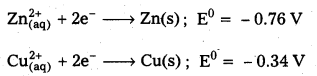
So, zinc will loss electrons to Cu2+ ions and redox reaction will occur as follows.
![]()
![]()
Question 17.
Write the cell reaction taking place in the cell, Cu(s) / Cu2+ (aq) //Ag+ (aq) / Ag (s).
Answer:
Cu(s)/Cu+2(aq)//Ag+(aq)/Ag
In the above notation copper electrode acts as anode and silver electrode acts as cathode.

Question 18.
Write the Nernst equation for the EMF of the cell
![]()
Answer:
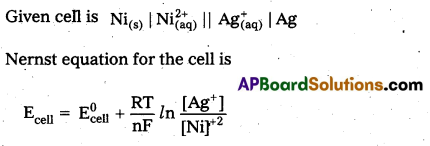
Question 19.
Write the cell reaction for which
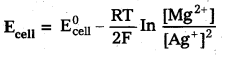
Answer:
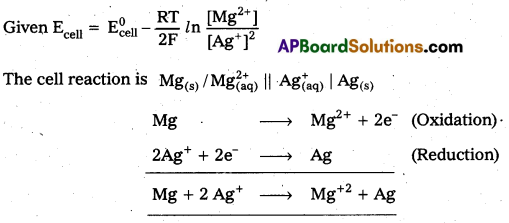
Short Answer Questions
Question 1.
What are galvanic cells ? Explain the working of a galvanic cell with a neat sketch taking Daniell cell as example.
Answer:
Galvanic cell: A device which converts chemical energy into electrical energy by the use of spontaneous redox reaction is called Galvanic cell (or) voltaic cell. Eg : Daniell cell.
Daniell cell: It is a special type of galvanic cell. It contains two half cells in the same vessel. The vessel is divided into two chambers. Left chamber is filled with ZnSO4 (aq) solution and Zn – rod is dipped into it. Right chamber is filled with aq. CuSO4 solution and a copper rod is dipped into it. Process diaphragm acts as Salt bridge. The two half cell’s are connected to external battery.
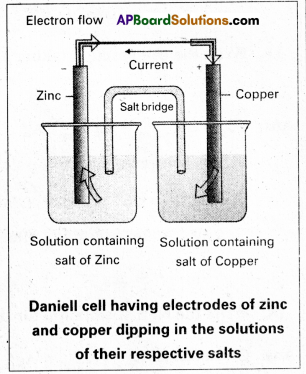
Cell reactions :
Ion Zn/ZnSO4 half cell, oxidation reaction occurs.
Zn → Zn2+ + 2e–
Ion Cu/CuSO4 half cell, reduction reaction occurs.
Cu+2 + 2e– → Cu
The net cell reaction is
Zn + Cu+2 \(\rightleftharpoons\) Zn+2 + Cu
Cell is represented as Zn / Zn+2 || Cu2+ / Cu
![]()
Question 2.
Give the construction and working of a standard hydrogen electrode with a neat diagram.
Answer:
To determine the potential of a single electrode experimentally, it is combined with a standard hydrogen electrode (electrode whose potential is known) and the EMF of the cell so constructed is measured with a potentiometer. Standard Hydrogen Electrode is constructed and is used as standard electrode or reference electrode. Standard Hydrogen Electrode (SHE) or Normal Hydrogen Electrode (NHE).
Pure hydrogen gas is bubbled into a solution of 1M HCl along a platinum electrode coated with platinum block. A platinum block electrode placed in the solution at atmospheric pressure.
Generally the electrode is fitted into the tube. The tube will have two circular small holes. This tube is immersed in the acid solution such that one half of the circular hole is exposed to air and another half in the solution.
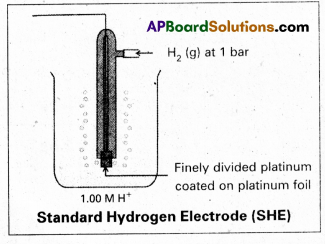
The following equilibrium exists at the electrode.
\(\frac{1}{2} \mathrm{H}_{2(\mathrm{~g})}\left(1_{\mathrm{atm}}\right) \rightleftharpoons \mathrm{H}_{(\mathrm{aq})}^{+}(\mathrm{M})+\mathrm{e}^{-}\)
Question 3.
State and explain Kohlrausch’s law of independent migration of ions. (IPF. 2015, BMP, 2016 (TS)
Answer:
Kohlrausch’s law of independent migration of ions : The limiting molar conductivity of the electrolytes can be represented as the sum of the individual contributions of the anion and the cation of the electrolytes.
\(\lambda_{\mathrm{m}(\mathrm{AB})}^0=\lambda_{\mathrm{A}^{+}}^0+\lambda_{\mathrm{B}^{-}}^0\)
\(\lambda_{\mathrm{m}}^0\) = Limiting molar conductivity
\(\lambda_{\mathrm{A}^{+}}^0\) = Limiting molar conductivity of cation
\(\lambda_{\mathrm{B}^{-}}^0\) = Limiting molar conductivity of anion,
Applications :
- Kohlrausch’s law is used in the calculation of the limiting molar conductivity of weak electrolytes.
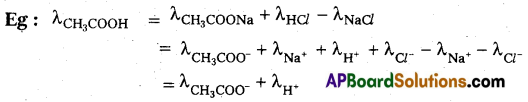
- This law is used in the calculation of degree of dissociation of a weak electrolyté.
- This law is used in the calculation of solubility of sparingly soluble salts like AgCl, BaSO4 etc.
![]()
Question 4.
What is electrolysis? Give Faraday’s first law of electrolysis. (IPE 2014)
Answer:
Electrolysis : The decomposition of a chemical compound in the molten state or in the solution state into its constituent elements under the influence of an applied EMF is called electrolysis.
The amount of chemical reaction which occurs at any electrode during electrolysis is proportional to the quantity of current passing through the electrolyte.
(Or)
The mass of the substance deposited at an electrode during the electrolysis of electrolyte is directly proportional to quantity of electricity passed through it.
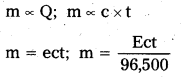
e = electrochemical equivalent
t = time in seconds
c = Current in amperes
E = Chemical equivalent
Question 5.
Calculate the emf of the cell at 25°C Cr | Cr3+ (0.1 M) || Fe2+ (0.01M)| Fe, given that
![]()
Answer:
Given cell is
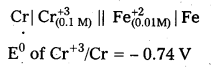
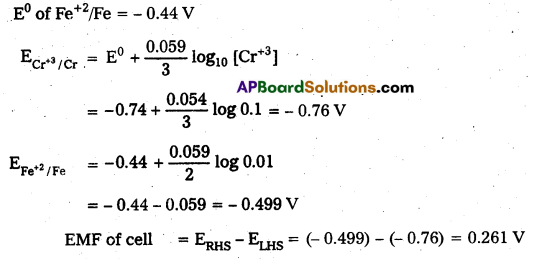
Question 6.
Determine the values of Kc for the following reaction.
![]()
Solution:
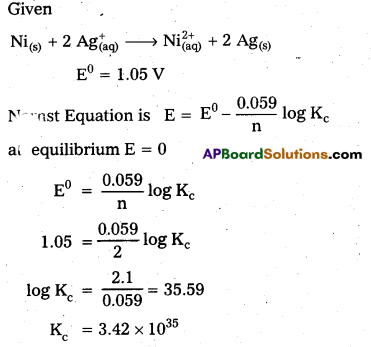
Question 7.
If a current of 0.5 ampere flows through a metallic wire for 2h, then how many electrons would flow through the wire ?
Solution:
Quantity of charge (Q) passed = Current (C) × Time (t)
= (0.5 A) × (2 × 60 × 60 s)
= (3600) Ampere sec = 3600 C
Number of electrons flowing through the wire on passing charge of one Faraday (96500 C) = 6.022 × 1023
Number of electrons flowing through the wire on passing a charge of 3600 C
= \(\frac{6.022 \times 10^{23} \times(3600 \mathrm{C})}{(96500 \mathrm{C})}\)
= 2.246 × 1022
Number of electrons = 2.246 × 1022
![]()
Question 8.
Define emf. Calculate the emf of the following galvanic cell:
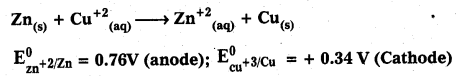
Solution:
EMF : The difference in electrode potentials between reduction potential of cathode and reduction potential of anode is called emf.
emf = reduction potential of cathode – reduction potential of anode
emf = + 0.34 – (-0.76) ⇒ + 0.34 + 0.76 = 1.1V
Question 9.
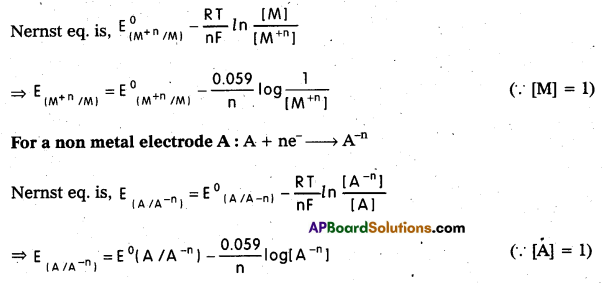
Write Nernst equation for a metal and non metal eletrode.
Solution:
For a metal electrode M : M+n (aq) + ne– → M(s)