Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material 8th Lesson Polymers Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material 8th Lesson Polymers
Very Short Answer Questions
Question 1.
Define the terms monomer and polymer.
Answer:
Monomer : The repeating structural units of polymer which are derived from simple and reactive molecules linked to one another by covalent bonds are called monomers.
Polymer : A large molecular weight complex compound which is formed by the.repeated combination of simple units (monomers) is called polymer.
![]()
Question 2.
What are polymers ? Give example.
Answer:
Polymer : A large molecular weight complex compound which is formed by the repeated combination of simple units (monomers) is called polymer.
E.g. : Nylon 6, 6, Buna-S,. rubber etc…..
Question 3.
What is polymerization ? Give an example of polymerization reaction.
Answer:
Polymerization: The process of formation of polymers from respective monomers is called polymerization.
(or)
A large molecular weight complex compound which is formed by the repeated combination of simple units is called polymer. This process is called polymerisation.
E.g. : Formation of polyethene from ethene and reaction of hexamethylene diamihe and adipic acid leading to the formation of Nylon 6, 6 are examples of two different types of polymerisation reactions.
Question 4.
Give one example each for synthetic and semi-synthetic polymers.
Answer:
Examples of synthetic polymers : Neoprene, Buna-S, Buna-N
Examples of Semi synthetic polymers : Cellulose rayon, cellulose nitrate.
![]()
Question 5.
How are the polymers classified on the basis of structure ?
Answer:
On the basis of structure, polymers are classified into three types :
- Linear polymers : These contains long and straight chains.
E.g.: PVC, polythene (high density) etc. - Branched chain polymers : These contains linear chains having some branches.
E.g.: low density polythene. - Cross linked polymers (or) Network polymers : These are usually formed from bi functional and tri functional monomers and contain strong covalent bonds between various linear polymer chains.
E.g.: Bakelite, melamine etc. .
Question 6.
Give one example each for linear and branched chain polymers.
Answer:
- Linear polymers : These contains long and straight chains.
E.g.: PVC, polythene (high density) etc. - Branched chain polymers : These contains linear chains having some branches.
E.g. : low density polythene.
Question 7.
What are cross linked (or network) polymers ? Give example.
Answer:
Cross linked polymers (or) Network polymers: These are usually formed from bi functional and tri functional monomers and contain strong covalent bonds between various linear polymer chains. E.g. : Bakelite, melamine etc.
Question 8.
What is addition polymer ? Give example. [T.S. Mar. 15]
Answer:
Addition Polymer: The polymer which is formed by the addition of molecules of monomers of same type (or) different type containing double bonds is called addition polymer.
E.g.: Polyethene, poly acrylonitrile.
![]()
Question 9.
What is condensation polymer ? Give example.
Answer:
Condensation polymer : The polymer which is formed by the condensation reaction between molecules having more than one functional group is called condensation polymer.
E.g.: Nylon 6, 6, Poly ethylene terephthalate.
Question 10.
What are homopolymers ? Give example.
Answer:
Homopolymers : The polymers which are formed by the polymerisation of a single monomeric species are known as homopolymers. E.g.: Polyethene, Poly styrene.
Question 11.
What are copolymers ? Give example.
Answer:
Copolymers : A polymer which is formed by the polymerisation of two (or) more chemically different types of monomer units is called copolymer. E.g.: Butadiene – Styrene polymer (Buna-S)
Question 12.
Is – [-CH2 – CH (C6H5)-]n – a homopolymer or a copolymer ?
Answer:
-[-CH2 – CH (C6H5-]n is polystyrene. It is a homopolymer.
It is formed by the polymerisation of single monomer styrene.
(C6H5 – CH = CH2)
![]()
Question 13.
Is (NH – CHR- CO)n a homopolymer or a copolymer ?
Answer:
[NH – CHR – CO]n is a homopolymer. It is formed by the polymerisation of single monomer a-amino acid (NH2 – CHR-COOH).
Question 14.
What are the classes of the polymer based on molecular forces ?
Answer:
On the basis of molecular forces polymers are classified into four types.
- Elastomers: These are rubber like solids with elastic properties.
E.g.: Buna-S, Buna-N. - Fibres : Fibres are the thread forming Solids which possess high tensile strength.
E.g. : Nylon 6, 6, polyesters - Thermoplastic Polymers : These are the linear (or) slightly branched long chain
molecules capable of softening on heating and hardening on cooling.
E.g.: Polystyrene, polythene. - Thermo setting polymers : These polymers are cross linked (or) heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
E.g. : Bakelite, urea-formaldehyde resin etc….
Question 15.
What are elastomers ? Give example. .
Answer:
Elastomers: These are rubber like solids with elastic properties. In elastomers the polymer chains are held together by the weak inter molecular forces.
E.g.: Buna – S, Buna – N etc.
Question 16.
What are fibres ? Give example.
Answer:
Fibres : Fibres are the thread forming solids which possess high tensile strength.
E.g.: Nylon 6, 6, polyesters
![]()
Question 17.
What are thermoplastic polymers ? Give example.
Answer:
Thermoplastic Polymers: These are the linear (or) slightly branched long chain molecules capable of softening on heating and hardening on cooling.
E.g.: Polystyrene, polythene.
Question 18.
What are thermosetting polymers ? Give example.
Answer:
Thermo setting polymers: These polymers are cross linked (or) heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
E.g.: Bakelite, urea-formaldehyde resin etc….
Question 19.
Write the name and structure of one of the common initiators used in free radical polymerization reaction.
Answer:
One of the common initiator used in free radical – polymerisation reaction is benzoyl peroxide.
Structure:
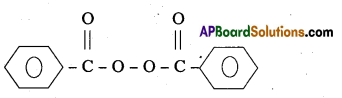
Question 20.
How can you differentiate between addition and condensation polymerization?
Answer:
Addition polymerisation
- Monomers used are unsaturated compounds.
- Polymer formation takes place without loss of atoms (or) groups.
- It is chain growth polymerisation.
- These polymers are called addition (or) ‘ chain (or) vinyl polymers.
Condensation polymerisation
- Monomers are bifunctional, tri-functional compounds.
- Polymer formation takes place with loss of atoms (or) groups like NH3, H20 etc.
- It is step growth polymerisation.
- These are called condensed polymers.
![]()
Question 21.
What is Ziegler – Natta catalyst? [T.S. Mar. 19]
Answer:
A mixture of Tri alkyl aluminium and titanium chloride is called Ziegler – Natta catalyst
E.g.: (C2H5)3 Al + TiCl4
Question 22.
How is Dacron obtained from ethylene glycol and terepthalic acid?
Answer:
Formation of dacron is an example of condensation polymerisation. It is obtained from ethylene glycol and terephthalic acid as follows.
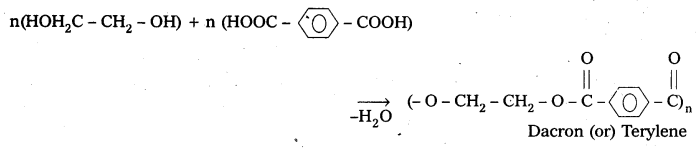
Question 23.
What are the repeating monomeric units of Nylon 6 and Nylon 6, 6?
Answer:
The repeating monomeric units of Nylon – 6 is Capro lactam
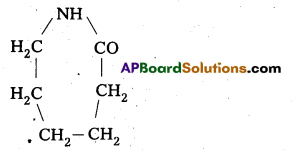
The repeating monomeric units of Nylon 6, 6 are hexamethylene diamine and Adipic acid.
H2N-(CH2)6 – NH2
Hexamethylene
HOOC – (CH2)4 – COOH
Adipic acid
Question 24.
What is the difference between Buna – N and Buna – S ?
Answer:
Buna – N : Buna – N is the copolymer which is formed by the polymerisation of 1, 3 – Butadiene and acrylonitrile.
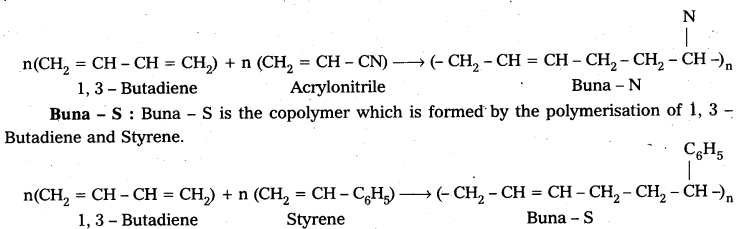
![]()
Question 25.
Arrange the following polymers in increasing order of their molecular forces.
- Nylon 6, 6 Buna – S, Polythene
- Nylon 6, Neoprene, Polyvinyl chloride
Answer:
- Increasing order of inter molecular forces of given polymers is
Buna – S < polythene < Nylon -6, 6 - Increasing order of inter molecular forces of given polymers is
Neoprene < polyvinyl chloride < Nylon – 6.
Question 26.
Identify the monomer in the following polymeric structures
![]()
Answer:
- Monomers present in
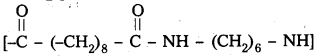 are decane dioic acid HOOC -CH2-)8 COOH and hexamethylene diamine H2N – (CH2-)6 NH2
are decane dioic acid HOOC -CH2-)8 COOH and hexamethylene diamine H2N – (CH2-)6 NH2 - Monomers present in (-NH – CO – NH – CH2 -)n are urea CO (NH2)2, formaldehyde HCHO.
Question 27.
Name the different types of molecular masses of polymers.
Answer:
The different types of important molecular masses of polymers are
- Number average molecular mass (\(\overline{\mathbf{M}}_{\mathrm{n}}\))
- Weight average molecular mass (\(\overline{\mathbf{M}}_{\mathrm{w}}\))
Question 28.
What is PDI (Poly Dispersity Index) ? [A.P. Mar. 19]
Answer:
Poly Dispersity Index (PDI) : The ratio between weight average molecular mass (\(\overline{\mathbf{M}}_{\mathrm{w}}\)) and the number average molecular mass (\(\overline{\mathbf{M}}_{\mathrm{n}}\)) of a polymer is called Poly Dispersity Index (PDI).
![]()
Question 29.
What is vulcanization of rubber ? [T.S. Mar. 19, 16; A.P. Mar. 17]
Answer:
Vulcanization of rubber : The process of heating the raw rubber with sulphur (or) with sulphur compounds to improve it’s physical properties is called vulcanization of rubber.
Question 30.
What is the cross linking agent used in the manufacture of type rubber ?
Answer:
In the manufacture of tyre rubber 5% sulphur is used as cross linking agent. ‘
Question 31.
What is biodegradable polymer ? Give one example of a biodegradable polyester ?
Answer:
Biodegradable poiymers : The polymers degradable by enzymatic hydrolysis and to some extent by oxidation are called biodegradable polymers.
E.g. : Nylon – 2, Nylon – 6, PHBV, Polyglycolic acid, Polylactic acid etc.
Question 32.
What is PHBV ? How is it useful to man ? [A.P. Mar. 19, 18, 16; T.S. Mar. 18, 16, 15]
Answer:
Poly β – hydroxy butyrate – CO – β – hydroxy Valerate (PHBV) : It is a Copolymer of 3 -hydroxy butanoic acid and 3 – hydroxy pentanoic acid.
Properties & Uses : The properties of PHBV vary according to the ratio of both the acids, 3-hydroxy butanoic acid provides stiffness and 3-hydroxy pentanoic acid imparts flexibility to copolymer.
It is used in medicine for making capsules.
PHBV also undergoes degradation by bacteria.
![]()
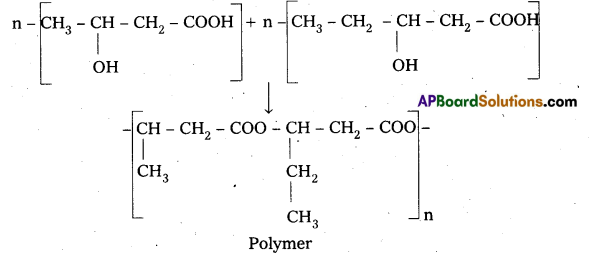
Question 33.
Give the structure of nylon 2 – nylon 6 ?
Answer:
Nylon 2 – Nylon 6 :
an alternating polyamide copolymer of glycine (![]() – COOH) and amino caproic acid (H2N – CH2)5 – COOH). It is a biodegradable polymer.
– COOH) and amino caproic acid (H2N – CH2)5 – COOH). It is a biodegradable polymer.
Structure of Nylon 2 – Nylon – 6
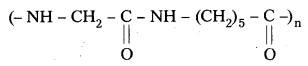
Short Answer Questions
Question 1.
Classify the following into addition and condensation polymers [A.P. Mar. 16]
i) Terylene
ii) Bakelite
iii) Polyvinyl chloride
iv) Polythene
Answer:
i) Terylene is a condensation polymer.
ii) Bakelite is a condensation polymer
iii) Polyvinyl chloride is an addition polymer
iv) Polythene is an addition polymer.
Question 2.
How do you explain the functionality of a polymer ?
Answer:
The number of bonding sites present in the monomers of the polymer is called functionality.
E.g.:
- Functionality of ethene, propene is one.
- Functionality of ethylene glycol is two.
![]()
Question 3.
Distinguish between the terms homo polymer and co polymer. Give one example of each.
Answer:
Homopolymers : The polymers which are formed by the polymerisation of a single monomeric species are known as homopolymers.
E.g. : Polyethene; Poly styrene.
Copolymers: A polymer which is formed by the polymerisation of two (or) more chemically different types of monomer units is called copolymer.
E.g.: Butadiene – Styrene polymer (Buna-S)
Question 4.
Define thermoplastics and thermosetting polymers with two examples of each.
Answer:
Thermoplastic Polymers : These are the linear (or) slightly branched long chain molecules capable of softening on heating and hardening on cooling. E.g. : Polystyrene, polythene.
Thermo setting polymers: These polymers are cross linked (or) heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
Eg.: Bakelite, urea-formaldehyd, resin etc….
Question 5.
Explain copolymerization with an example.
Answer:
Copolymers: A polymer which is formed by the polymerisation of two (or) more chemically different types of monomer units is called copolymer.
E.g.: Butadiene-Styrene polymer(Buna-S)
The process of formation of copolymer is called copolymerisation.
E.g. : Buna – S : Buna – S is the copolymer which is formed by the polymerisation of 1,3- Butadiene and Styrene.
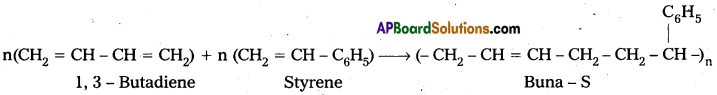
Question 6.
Explain free radical mechanism for the polymerization of ethene.
Answer:
Polymerisation of ethene to polythene proceeds through free radical mechanism. The initiator used in this reaction is benzoyl peroxide. This mechanism involves three steps.
1) Chain initiation : The addition of phenyl free radical formed by the peroxide to the ethene double bond and forms a larger free radical.
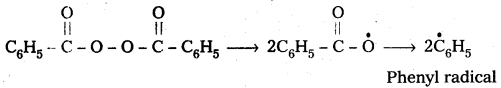
C6H5 + CH2 = CH2 → C6H5 – CH2 – CH2
2) Chain propagation : The above formed radical reacts with another molecule of ethene another bigger sized radical is formed. The repetetion of this sequence takes places.
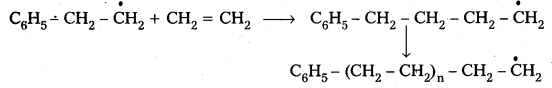
3) Chain Termination : The product radical formed reacts with another radical to form polymerised product.
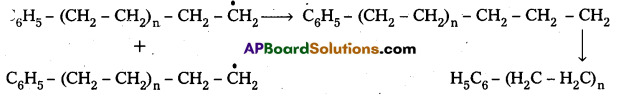
![]()
Question 7.
Write the names and structures of the monomers used for getting the following polymers . [Mar. 14]
i) Polyvinyl chloride
ii) Teflon
iii) Bakelite
iv) Polystyrene.
Answer:
i) Polyvinyl chloride
Monomer : Vinyl chloride
Structure : CH2 = CH – Cl
ii) Teflon
Monomer : Tetrafluoro ethylene
Structure : CF2 = CF2
iii) Bakelite
Monomers : Phenol, Formal dehyde
Structure : 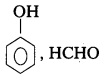
iv) Polystyrene
Monomer : Styrene
Structure : 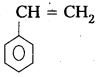
Question 8.
Write the names and structures of the monomers of the following polymers.
i) Buna – S
ii) Buna – N
iii) Dacron
iv) Neoprene
Answer:
i) Buna – S
Monomers : 1,3- Butadiene, Styrene
Structure :
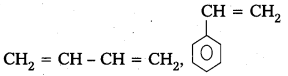
ii) Buna – N
Monomers : 1,3- Butadiene, Acrylonitrile
Structure : CH2 = CH – CH = CH2, CH2 = CH – CN
iii) Dacron
Monomers : Ethylene glycol, Terephthalic acid
Structure : HOCH2 – CH2OH, C HOOC ![]() COOH
COOH
iv) Neoprene
Monomers : 2 – chloro -1,3- Butadiene
Structure :
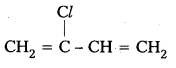
![]()
Question 9.
What is natural rubber ? How does it exhibit elastic properties ?
Answer:
- Natural rubber is a polymer and possesses elastic properties.
- It is an elastomer and it is manufactured from rubber latex. Latex is a colloidal dispersion of rubber in water.
- Natural rubber may be considered as a linear polymer of isoprene. It is also called as cis-1, 4-Poly isoprene.
- The cis poly isoprene molecule consists of various chains held together by weak vander waals interactions and has a colloid structure. Thus it stretches like a spring and exhibits elastic properties.
Question 10.
Explain the purpose of vulcanization of rubber. [T.S. Mar. 17]
Answer:
- Natural rubber becomes soft at high temperatures and brittle at low temperatures. It shows high water absorption capacity.
- Natural rubber is soluble in non-polar solvents and is non-resistant to oxidising agents.
- To improve these physical properties rubber can be vulcanised.
- The process of heating the raw rubber with sulphur (or) with sulphur compounds to improve it’s physical properties is called vulcanisation of rubber.
- The vulcanized rubber has improved properties like elasticity, minimum water absorbing tendency, high resistance to chemical oxidation as well as organic solvents.
Question 11.
Explain the difference between natural rubber and synthetic rubber.
Answer:
Natural rubber : The rubber which is obtained from natural sources such as plants and animals rubber called natural rubber. E.g.: Starch, cellulose, rubber etc.
Synthetic rubber : The rubber which are artificially prepared i.e. man-made are called synthetic rubbers.
These have wide applications in daily life as well as in industry.
![]()
Question 12.
How does the presence of double bonds in rubber molecules influence their structure and reactivity ?
Answer:
- Natural rubber is cis. poly isoprene. It is obtained by polymerisation of isoprene. The polymerisation takes place at 1, 4 positions. .
- In rubber molecule double bonds are located between C2 and C3 of the monomer
isoprene. - These double bonds (cis) do not allow the polymer chain to come closer. Therefore only weak vander waal’s forces are present.
- The chains are not linear, they can be stretched just like springs and exhibits elastic properties.
Question 13.
What are LDP and HDP ? How are they formed ?
Answer:
Polythenes are two types : .
- LDP (Low Density Polythene),
- HDP (High Density Polythene)
1) Low Density Polythene : LDP is formed by the polymerisation of ethene under high pressure of 1000 to 2000 atm. at a pressure of 350 to 570K in the presence of traces of dioxygen (or) a peroxide initiator.
Properties :
a) This is obtained through the free radical addition.
b) LDP is chemically inert and tough.
c) LDP is flexible and a poor conductor of electricity.
Uses:
a) It is used in the insulation of electric cables.
b) It is used in the manufacture of pipes in agriculture irrigation.
2) High Density Polythene : HDP is formed by the polymerisation of ethene in a hydro carbon solvent in presence of Ziegler Natta catalyst at a temperature of’333K to 343 K and under a pressure of 6 – 7 atm
Properties :
a) HDP consists of linear molecules and has high density due to close packing.
b) It is chemically inert and more tough, hard.
Uses:
a) It is used in manufacture of house hold articles like buckets, dustbins etc.
b) It is used in manufacture of pipes.
Question 14.
What are natural and synthetic polymers ? Give two examples of each type.
Answer:
Natural polymers : The polymers which are obtained from natural sources such as plants and animals are called natural polymers.
E.g.: Starch, cellulose, rubber etc.
Synthetic polymers: The polymers which are artificially prepared i.e. man-made are called synthetic polymers.
These have wide applications in daily life as well as in industry.
E.g.: Plastics, Nylon 6, 6, synthetic rubbers.
![]()
Question 15.
Write notes on different types molecular masses of polymers.
Answer:
The molecular mass of the polymer doesnot remain constant as in the case of simple chemical substances. So the molecular weight of a polymer is expressed in terms Of “average value”.
The average molecular masses of polymers expressed in different ways. The following are the important among them.
- Number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\)),
- Weight average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{w}}\))
i) Number average molecular mass (\(\overline{\mathrm{M}}_{\mathrm{n}}\)) : If a polymer sample contains N1 particles of mass M1 each and N2 particles of Mass M2 each and so on.
The number average molecular weight (\(\overline{\mathrm{M}}_{\mathrm{n}}\)) of the polymer = \(\frac{\sum_{\mathrm{n}_{\mathrm{i}}=1}^{\infty} \mathrm{N}_{\mathrm{i}} \mathrm{M}_{\mathrm{i}}}{\sum_{\mathrm{n}_{\mathrm{i}}=1}^{\infty} \mathrm{N}_{\mathrm{i}}}\)
(\(\overline{\mathrm{M}}_{\mathrm{n}}\)) can be determined by end group analysis method (or) physical methods based on the number of particle present.
ii) Weight average molecular weight (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) : Weight average molecular weight (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) is calculated by multiplying the molecular weight of each type of particle with contribution of the species to the total weight of the sample.
Weight average molecular weight (\(\overline{\mathrm{M}}_{\mathrm{w}}\)) of the polymer = \(\frac{\sum_{n_{i}=1}^{\infty} N_{i} M_{i}^{2}}{\sum_{n_{i}=1}^{\infty} N_{i} M_{i}}\)
Long Answer Questions
Question 1.
Write an essay on i) Addition polymerization and ii) Condensation polynerization
Answer:
i) Addition Polymerization : Addition polymerization involves the self addition of unsaturated monomers without loss of any small molecules to form polymer.
The polymer so formed is called addition polymer.
This polymerisation is also called chain growth polymerisation.
In addition polymerisation, monomers should contain double bonds.
This type of polymerisation can be broadly subdivided into 2 groups.
a) ionic polymerisation (cationic & anionic)
b) free radical polymerization
Addition polymerization mechanism involves three steps. They are : i)-Chain initiation step ii) Chain propagation step and iii) Chain termination step.
E.g. : Vinyl Chloride molecules polymerises to PVC.
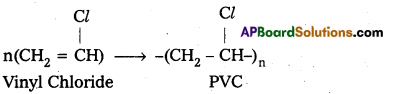
ii) Condensation polymerization : In this polymerisation monomers combine together with the loss of simple molecules like H2O, NH3 etc. The formed polymer is called condensation polymer.
Condensation polymers are generally derived from di (or) polyfunctional monomers. This polymerization is also called step polymerization.
E.g.: i) Nylon – 6,6 is formed from hexamethylene diamine and adipic acid by condensation.
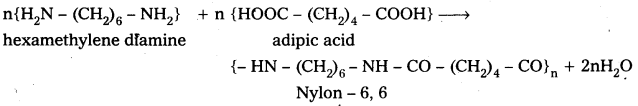
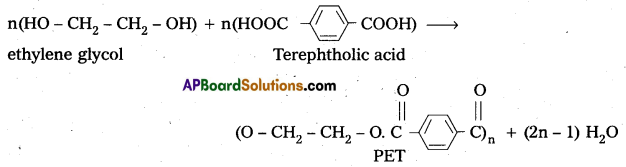
ii) Polyethylene terephthalate (PET) from ethylene glycol and terephtholic acid by condensation reaction.
Question 2.
Explain the classification of polymers based on their source and structure.
Answer:
On the basis of source polymers are classified into three types.
- Natural polymers
- Semi synthetic polymers
- Synthetic polymers
1) Natural polymers : The polymers which are obtained from natural sources such as plants and animals are called natural polymers.
E.g.: Starch, cellulose, rubber etc.
2) Semi synthetic polymers : The polymers which are synthetic derivatives of the natural polymers are called semisynthetic polymers.
E.g. : Cellulose rayon, cellulose nitrate.
3) Synthetic polymers : The polymers which are artificially prepared i.e. man-made are called synthetic polymers. .
These have wide applications in daily life as well as in’ industry.
E.g.: Plastics, Nylon 6, 6, synthetic rubbers.
Classification of polymers on the basis of structure :
i) Linear polymers : It is one in which the repeating units are similar to the links in a very long chain.
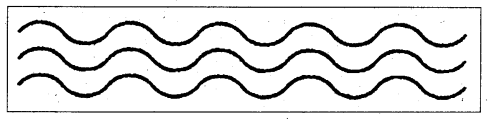
E.g.: Polythene, PVC etc.
ii) Branched chain polymers : It is one in which some of the molecules are attached as side chains to the linear chains.
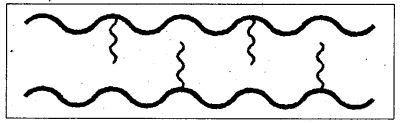
E.g.: Low density polythene (LDPE) etc.
iii) Cross linked (or) network polymers : More branching at random points connecting many chains give rise to network (or) cross linked polymers.
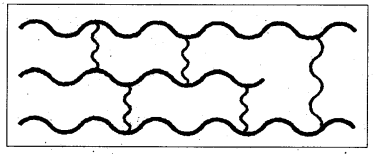
E.g.: Bakelite, melamine etc.
![]()
Question 3.
Explain the classification of polymers based on the mode of polymerization and nature of molecular forces.
Answer:
On the basis of mode of polymerisation polymers are classified into two types :
1) Addition polymers
2) Condensation polymers.
1) Addition Polymer : The polymer which is formed by the addition of molecules of monomers of same type (or) different type containing double bonds is called addition polymer.
Eg.: Polyethene, poly acrylonitrile.
2) Condensation polymer : The polymer which is formed by the condensation reaction between molecules having more than one functional group is called condensation polymer.
Eg.: Nylon 6, 6, Poly Ethylene terephthalate.
On the basis of molecular forces polymers are classified into four types.
- Elastomers : These are rubber like solids with elastic properties.
Eg.: Buna-S, Buna-N. - Fibres : Fibres are the thread forming solids which possess high tensile strength.
Eg.: Nylon 6, 6, polyesters. - Thermoplastic Polymers : These are the linear (or) slightly branched long chain molecules capable of softening on heating and hardening on cooling.
Eg.: Polystyrene, polythene. - Thermo setting polymers : These polymers are cross linked (or) heavily branched molecules which on heating undergo extensive cross linking in moulds and again become infusible.
Eg.: Bakelite, urea-formaldehyde resin etc….
Question 4.
What are synthetic rubbers ? Explain the preparation and uses of the following
i) Neoprene
ii) Buna-N
iii) Buna-S.
Answer:
The polymer which is capable of getting stretched almost to double the length and which is also vulcanizable as in the case of natural rubber is called a synthetic rubber.
1) These are homo polymers of 1, 3 – butadiene derivatives (or) copolymers in which one of the monomer is 1, 3 – Butadiene (or) its derivatives and the other is any unsaturated monomer.
2) Synthetic rubber return to its original shape and size when the stretched force is removed.
Eg.: Neoprene, Buna – S, Buna – N.
i) Neoprene : Neoprene is formed by the free radical polymerisation of chloroprene (2-Chloro 1, 3 – Butadiene)
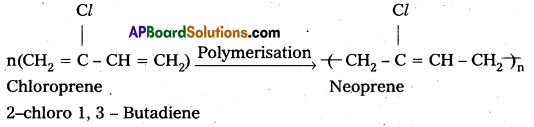
Uses:
1) It has superior resistance to vegetable and animal oils.
2) It is used in the manufacture of gaskets, conveyor belts.
ii) Buna – N : Buna – N is the copolymer which is formed by the polymerisation of 1, 3 – Butadiene and acrylonitrile.
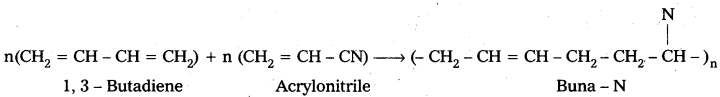
Uses:
1) It is resistant to the action of petrol, lubricating oil and organic solvents.
2) It is used in. making oil seals, tank lining.
iii) Buna – S : Buna – S is the copolymer which is formed by the polymerisation of 1, 3 – Butadiene and Styrene.
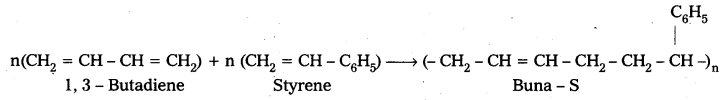
Uses :
1) It is a good substitute for natural rubber.
2) It is used in manufacture of auto tyres.
3) It is used in manufacture of floor tiles.
4) It is used in manufacture of foot wear components, cable insultation
Textual Examples
Question 1.
Is [-CH2 – CH (C6H5-)] n a homopolymer or a copolymer ?
Solution:
It is a homopolymer and the monomer from which it is obtained is styrene C6H5CH = CH2.
![]()
Question 2.
A polymer contains 10 molecules with molecular mass 10,000 and 10 molecules with molecular mass 1,00,000. Calculate number – average molecular mass.
Solution:
\(\overline{\mathrm{M}}_{\mathrm{n}}=\frac{\sum \mathrm{N}_{\mathrm{i}} \mathrm{M}_{\mathrm{i}}}{\sum \mathrm{N}_{\mathrm{i}}}=\frac{10 \times 10000+10 \times 100000}{10+10}\) = 55,000
Intext Questions
Question 1.
What are polymers ?
Answer:
Polymers are high molecular mass substances consisting of large -number of repeating structural units. They are also called as macromolecules. Some examples of polymers are polythene, bakelite, rubber, nylon 6, 6 etc.
Question 2.
How are polymers classified on the basis of structure ?
Answer:
On the basis of structure, the polymers are classified as below :
- Linear polymers such as polythene, polyvinyl chloride, etc.
- Branched chain polymers such as low density polythene.
- Cross linked polymers such as bakelite, melamine, etc.
Question 3.
Write the names of monomers of the following polymers.
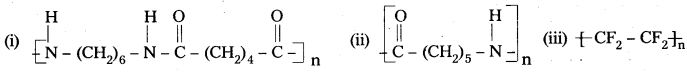
Answer:
- Hexamethylene diamine and adipic acid
- Caprolactam
- Tetrafluoroethene.
![]()
Question 4.
Classify the following as addition and condensation polymers : Terylene, Bakelite, Polyvinyl chloride, polythene.
Answer:
Additon polymers : Polyvinyl chloride, polythene. Condensation polymers : Terylene, Bakelite.
Question 5.
Explain the difference between Buna – N and Buna – S. .
Answer:
Buna – N is a copolymer of 1, 3 – butadiene and acrylonitrile and Buna-S a copolymer of 1, 3—butadiene and styrene.
Question 6.
Arrange the following polymers in increasing order of their intermolecular forces.
i) Nylon 6, 6 Buna — S, Polythene. ii) Nylon 6, Neoprene, Polyvinyl chloride.
Answer:
In order of increasing intermolecular forces.
- Buna—S, Polythene, Nylon 6, 6.
- Neoprene, Polyvinyl chloride, Nylon 6.