Telangana & Andhra Pradesh BIEAP TS AP Intermediate Inter 2nd Year Chemistry Study Material Textbook Solutions Guide PDF Free Download, TS AP Inter 2nd Year Chemistry Blue Print Weightage 2022-2023, Telugu Academy Intermediate 2nd Year Chemistry Textbook Pdf Download, Questions and Answers Solutions in English Medium and Telugu Medium are part of AP Inter 2nd Year Study Material Pdf.
Students can also read AP Inter 2nd Year Chemistry Syllabus & AP Inter 2nd Year Chemistry Important Questions for exam preparation. Students can also go through AP Inter 2nd Year Chemistry Notes to understand and remember the concepts easily.
AP Intermediate 2nd Year Chemistry Study Material Pdf Download | Sr Inter 2nd Year Chemistry Textbook Solutions
AP Inter 2nd Year Chemistry Study Material in English Medium
- Chapter 1 Solid State
- Chapter 2 Solutions
- Chapter 3 Electrochemistry and Chemical Kinetics
- Chapter 4 Surface Chemistry
- Chapter 5 General Principles of Metallurgy
- Chapter 6 P-Block Elements
- Chapter 7 d and f Block Elements & Coordination Compounds
- Chapter 8 Polymers
- Chapter 9 Biomolecules
- Chapter 10 Chemistry in Everyday Life
- Chapter 11 Haloalkanes and Haloarenes
- Chapter 12 Organic Compounds Containing C, H and O
- Chapter 13 Organic Compounds Containing Nitrogen
AP Inter 2nd Year Chemistry Study Material in Telugu Medium
- Chapter 1 ఘనస్థితి
- Chapter 2 ద్రావణాలు
- Chapter 3 విద్యుత్ రసాయనశాస్త్రం – రసాయన గతికశాస్త్రం
- Chapter 4 ఉపరితల రసాయనశాస్త్రం
- Chapter 5 లోహనిష్కర్షణలో సాధారణ సూత్రాలు
- Chapter 6 p-బ్లాకు మూలకాలు
- Chapter 7 d,f – బ్లాక్ మూలకాలు & సమన్వయ సమ్మేళనాలు
- Chapter 8 పాలిమర్ లు
- Chapter 9 జీవాణువులు
- Chapter 10 నిత్యజీవితంలో రసాయనశాస్త్రం
- Chapter 11 హాలో ఆల్కేన్లు, హాలో ఎరీస్లు
- Chapter 12 C,H,O లు ఉన్న కర్బన సమ్మేళనాలు
- Chapter 13 నైట్రోజన్లో ఉన్న కర్బన సమ్మేళనాలు
TS AP Inter 2nd Year Chemistry Weightage Blue Print 2022-2023
TS AP Inter 2nd Year Chemistry Weightage 2022-2023 | TS AP Inter 2nd Year Chemistry Blue Print 2022
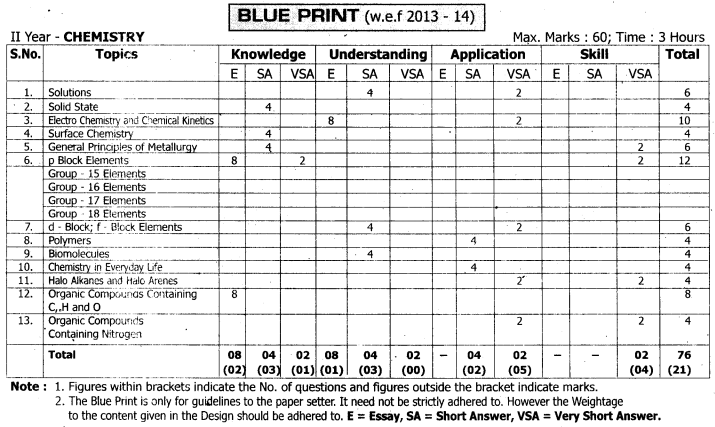
Intermediate 2nd Year Chemistry Syllabus
TS AP Inter 2nd Year Chemistry Syllabus
Chapter 1 Solid State
- 1.1 General characteristics of solid state
- 1.2 Amorphous and crystalline solids
- 1.3 Classification of crystalline solids based on different binding forces (molecular, ionic, metallic, and covalent solids)
- 1.4 Probing the structure of solids: X-ray crystallography
- 1.5 Crystal lattices and unit cells, Bravais lattices primitive and centered unit cells
- 1.6 Number of atoms in a unit cell (primitive, body-centered, and face-centered cubic unit cell)
- 1.7 Close packed structures: Close packing in one dimension, in two dimensions, and in three dimensions- tetrahedral and octahedral voids- formula of a compound and number of voids filled- locating tetrahedral and octahedral voids
- 1.8 Packing efficiency in simple cubic, bcc, and in hcp, ccp lattice.
- 1.9 Calculations involving unit cell dimensions- density of the unit cell.
- 1.10 Imperfections in solids-types of point defects-stoichiometric and non-stoichiometric defects
- 1.11 Electrical properties-conduction of electricity in metals, semiconductors, and insulators band theory of metals
- 1.12 Magnetic properties
Chapter 2 Solutions
- 2.1 Types of solutions
- 2.2 Expressing concentration of solutions-mass percentage, volume percentage, mass by volume percentage, parts per million, mole fraction, molarity, and molality
- 2.3 Solubility: Solubility of a solid in a liquid, solubility of a gas in a liquid, Henry’s law
- 2.4 Vapour pressure of liquid solutions: vapour pressure of liquid-liquid solutions. Raoult’s law is a special case of Henry’s law – vapour pressure of solutions of solids in liquids
- 2.5 Ideal and non-ideal solutions
- 2.6 Colligative properties and determination of molar mass-relative lowering of vapour pressure-elevation of boiling point-depression of freezing point-osmosis and osmotic pressure-reverse osmosis and water purification.
- 2.7 Abnormal molar masses-van’t Hoff factor
Chapter 3 Electrochemistry And Chemical Kinetics
Electrochemistry
- 3.1 Electrochemical cells
- 3.2 Galvanic cells measurement of electrode potentials
- 3.3 Nernst equation-equilibrium constant from Nernst equation-electrochemical cell and Gibbs energy of the cell reaction
- 3.4 Conductance of electrolytic solutions- measurement of the conductivity of ionic solutions- a variation of conductivity and molar conductivity with concentration-strong electrolytes and weak electrolytes-applications of Kohlrausch’s law
- 3.5 Electrolytic cells and electrolysis: Faraday’s laws of electrolysis-products of electrolysis
- 3.6 Batteries: primary batteries and secondary batteries
- 3.7 Fuel cells
- 3.8 Corrosion of metals-Hydrogen economy
Chemical Kinetics
- 3.9 Rate of a chemical reaction
- 3.10 Factors influencing the rate of a reaction: dependence of rate on concentration- rate expression and rate constant- order of a reaction, molecularity of a reaction
- 3.11 Integrated rate equations-zero order reactions-first order reactions-half life of a reaction
- 3.12 Pseudo first-order reaction
- 3.13 Temperature dependence of the rate of a reaction-effect of catalyst
- 3.14 Collision theory of chemical reaction rates
Chapter 4 Surface Chemistry
- 4.1 Adsorption and absorption: Distinction between adsorption and absorption-mechanism of adsorption-types of adsorption- characteristics of physisorption- characteristics of chemisorptions- adsorption isotherms- adsorption from solution phase- applications of adsorption
- 4.2 Catalysis: Catalysts, promoters, and poisons-auto catalysis- homogeneous and heterogeneous catalysis- adsorption theory of heterogeneous catalysis- important features of solid catalysts: (a) activity (b) selectivity- shape-selective catalysis by zeolites- enzyme catalysis- characteristics and mechanism- catalysts in industry
- 4.3 Colloids
- 4.4 Classification of colloids: Classification based on the physical state of the dispersed phase and dispersion medium- classification based on nature of the interaction between the dispersed phase and dispersion medium- classification based on the type of particles of the dispersed phase- multi molecular, macromolecular, and associated colloids-cleansing action of soaps-preparation of colloids-purification of colloidal solutions-properties of colloidal solutions: Tyndal effect, colour, Brownian movement-charge on colloidal particles, electrophoresis
- 4.5 Emulsions
- 4.6 Colloids Around us- application of colloids
Chapter 5 General Principles of Metallurgy
- 5.1 Occurance of metals
- 5.2 Concentration of ores- levigation, magnetic separation, froth floatation, leaching
- 5.3 Extraction of crude metal from concentrated ore-conversion to oxide, reduction of oxide to the metal
- 5.4 Thermodynamic principles of metallurgy-Ellingham diagram-limitations-applications-extraction of iron, copper, and zinc from their oxides
- 5.5 Electrochemical principles of metallurgy
- 5.6 Oxidation and reduction
- 5.7 Refining of crude metal distillation, liquation poling, electrolysis, zone refining, and vapour phase refining
- 5.8 Uses of aluminium, copper, zinc, and iron
Chapter 6 P-Block Elements
Group-15 Elements
- 6.1 Occurance- electronic configuration, atomic and ionic radii, ionization energy, electronegativity, physical and chemical properties
- 6.2 Dinitrogen- preparation, properties, and uses
- 6.3 Compounds of nitrogen-preparation and properties of ammonia
- 6.4 Oxides of nitrogen
- 6.5 Preparation and properties of nitric acid
- 6.6 Phosphorous-allotropic forms
- 6.7 Phosphine-preparation and properties
- 6.8 Phosphorous halides
- 6.9 Oxoacids of phosphorous
Group-16 Elements
- 6.10 Occurance- electronic configuration, atomic and ionic radii, ionization enthalpy, electron gain enthalpy, electronegativity, physical and chemical properties
- 6.11 Dioxygen-preparation, properties, and uses
- 6.12 Simple oxides
- 6.13 Ozone preparation, properties, structure and uses
- 6.14 Sulphur-allotropic forms
- 6.15 Sulphur dioxide preparation, properties and uses
- 6.16 Oxoacids of sulphur
- 6.17 Sulphuric acid-industrial process of manufacture, properties and uses
Group-17 Elements
- 6.18 Occurance, electronic configuration, atomic and ionic radii, ionization enthalpy, electron gain enthalpy, electronegativity, physical and chemical properties
- 6.19 Chlorine preparation, properties, and uses
- 6.20 Hydrogen chloride- preparation, properties and uses
- 6.21 Oxoacids of halogens
- 6.22 Interhalogen compounds
Group-18 Elements
- 6.23 Occurance, electronic configuration, ionisation enthalpy,atomic radii electron gain enthalpy, physical and chemical properties (a) Xenon-fluorine compounds – XeF2, XeF4 and XeF6 – preparation,hydrolysis and formation of fluoro anions- structures of XeF2, XeF4 and XeF6 (b) Xenon-oxygen compounds XeO3 and XeOF4 – their formation and structures
Chapter 7 d and f Block Elements & Coordination Compounds
d And f Block Elements
- 7.1 Position in the periodic table
- 7.2 Electronic configuration of the d-block elements
- 7.3 General properties of the transition elements (d-block) -physical properties, variation in atomic and ionic sizes of transition series, ionization enthalpies, oxidation states, trends in the M2+/M and M3+/M2+ standard electrode potentials, trends in the stability of higher oxidation states, chemical reactivity and EJ values, magnetic properties, formation of colored ions, formation of complex compounds, catalytic properties, formation of interstitial compounds, alloy formation
- 7.4 Some important compounds of transition elements-oxides and oxoanions of metals-preparation and properties of potassium dichromate and potassium permanganate-structures of chromate, dichromate, manganate, and permanganate ions
- 7.5 Inner transition elements(f-block)-lanthanoids- electronic configuration-atomic and ionic sizes-oxidation states- general characteristics
- 7.6 Actinoids-electronic configuration atomic and ionic sizes, oxidation states, general characteristics, and comparison with lanthanoids
- 7.7 Some applications of d and f block elements
Coordination Compounds
- 7.8 Werner’s theory of coordination compounds
- 7.9 Definitions of some terms used in coordination compounds
- 7.10 Nomenclature of coordination compounds- IUPAC nomenclature
- 7.11 Isomerism in coordination compounds-(a)Stereo isomerism- Geometrical and optical isomerism (b)Structural isomerism- linkage, coordination, ionization, and solvate isomerism
- 7.12 Bonding in coordination compounds. (a)Valence bond theory – magnetic properties of coordination compounds-limitations of valence bond theory (b) Crystal field theory (i) Crystal field splitting in octahedral and tetrahedral coordination entities (ii) Colour in coordination compounds-limitations of crystal field theory
- 7.13 Bonding in metal carbonyls
- 7.14 Stability of coordination compounds
- 7.15 Importance and applications of coordination compounds
Chapter 8 Polymers
- 8.1 Classification of Polymers -Classification based on source, structure, mode of polymerization, molecular forces, and growth polymerization
- 8.2 Types of polymerization reactions- addition polymerization or chain growth polymerization- ionic polymerization, free radical mechanism-preparation of addition polymers- polythene, Teflon, and polyacrylonitrile-condensation polymerization or step growth polymerization-polyamides- preparation of Nylon 6,6 and nylon 6-poly esters- terylene- bakelite, melamine, formaldehyde polymer- copolymerization- Rubber- natural rubber-vulcanization of rubber-Synthetic rubbers- preparation of neoprene and buna-N
- 8.3 Molecular mass of polymers-number average and weight average molecular masses- polydispersity index(PDI)
- 8.4 Biodegradable polymers- PHBV, Nylon 2-nylon 6
- 8.5 Polymers of commercial importance- poly propene, polystyrene, polyvinyl chloride(PVC), urea-formaldehyde resin, glyptal, bakelite- their monomers, structures, and uses
Chapter 9 Biomolecules
- 9.1 Carbohydrates – Classification of carbohydrates- Monosaccharides: preparation of glucose from sucrose and starch- Properties and structure of glucose- D, L and (+), (-) configurations of glucose- Structure of fructose Disaccharides: Sucrose- preparation, structure-Invert sugar- Structures of maltose and lactose-Polysaccharides: Structures of starch cellulose and glycogen- Importance of carbohydrates
- 9.2 Aminoacids: Natural amino acids- classification of amino acids – structures and D and L forms-Zwitter ions Proteins: Structures, classification, fibrous and globular- primary, secondary, tertiary, and quaternary structures of proteins- Denaturation of proteins
- 9.3 Enzymes: Enzymes, mechanism of enzyme action
- 9.4 Vitamins: Explanation- names- classification of vitamins – sources of vitamins-deficiency diseases of different types of vitamins
- 9.5. Nucleic acids: chemical composition of nucleic acids, structures of nucleic acids, DNA fingerprinting biological functions of nucleic acids
- 9.6 Hormones: Definition, different types of hormones, their production, biological activity, diseases due to their abnormal activities.
Chapter 10 Chemistry in Everyday Life
- 10.1 Drugs and their classification: (a) Classification of drugs on the basis of pharmacological effect(b) Classification of drugs on the basis of drug action (c) Classification of drugs on the basis of chemical structure (d) Classification of drugs on the basis of molecular targets
- 10.2 Drug-Target interaction-Enzymes as drug targets (a) Catalytic action of enzymes (b) Drug-enzyme interaction Receptors as drug targets
- 10.3 Therapeutic action of different classes of drugs: antacids, antihistamines, neurologically active drugs: tranquilizers, analgesics- non-narcotic, narcotic analgesics, antimicrobials-antibiotics, antiseptics, and disinfectants- antifertility drugs
- 10.4 Chemicals in food- artificial sweetening agents, food preservatives, antioxidants in food
- 10.5 Cleansing agents-soaps and synthetic detergents
Chapter 11 Halo Alkanes and Halo Arenes
- 11.1 Classification and nomenclature
- 11.2 Nature of C-X bond
- 11.3 Methods of preparation: Alkyl halides and aryl halides- from alcohols, from hydrocarbons (a) by free radical halogenation (b) by electrophilic substitution (c) by replacement of diazonium group(Sand- Meyer reaction) (d) by the addition of hydrogen halides and halogens to alkenes-by halogen exchange(Finkelstein reaction)
- 11.4 Physical properties- melting and boiling points, density and solubility
- 11.5 Chemical reactions: Reactions of haloalkanes (i)Nucleophilic substitution reactions (a) SN2 mechanism (b) SN1 mechanism (c) stereochemical aspects of nucleophilic substitution reactions -optical activity (ii) Elimination reactions (iii) Reaction with metals-Reactions of haloarenes: (i) Nucleophilic substitution (ii)Electrophilic substitution and (iii) Reaction with metals
- 11.6 Polyhalogen compounds: Uses and environmental effects of dichloro methane, trichloromethane, triiodomethane, tetrachloro methane, freons, and DDT.
Chapter 12 Organic Compounds Containing C, H, and O (Alcohols, Phenols, Ethers, Aldehydes, Ketones, and Carboxylic Acids)
Alcohols, Phenols, and Ethers
- 12.1 Alcohols, phenols and ethers-classification
- 12.2 Nomenclature: (a) Alcohols, (b) phenols and (c) ethers
- 12.3 Structures of hydroxy and ether functional groups
- 12.4 Methods of preparation: Alcohols from alkenes and carbonyl compounds- Phenols from haloarenes, benzene sulphonic acid, diazonium salts, cumene
- 12.5 Physical properties of alcohols and phenols
- 12.6 Chemical reactions of alcohols and phenols (i) Reactions involving cleavage of O-H bond-Acidity of alcohols and phenols, esterification (ii) Reactions involving cleavage of C-O bond- reactions with HX, PX3, dehydration, and oxidation (iii) Reactions of phenols- electrophilic aromatic substitution, Kolbe’s reaction, Reimer – Tiemann reaction, reaction with zinc dust, oxidation
- 12.7 Commercially important alcohols (methanol, ethanol)
- 12.8 Ethers-Methods of preparation: By dehydration of alcohols, Williamson synthesis-Physical properties-Chemical reactions: Cleavage of C-O bond and electrophilic substitution of aromatic ethers.
Aldehydes and Ketones
- 12.9 Nomenclature and structure of carbonyl group 12.10Preparation of aldehydes and ketones-(1) by oxidation of alcohols (2) by dehydrogenation of alcohols (3) from hydrocarbons
- 12.10 Preparation of aldehydes (1) from acyl chlorides (2) from nitriles and esters (3) from hydrocarbons-Preparation of ketones (1) from acyl chlorides (2) from nitriles (3) from benzene or substituted benzenes
- 12.11 Physical properties of aldehydes and ketones
- 12.12 Chemical reactions of aldehydes and ketones-nucleophilic addition, reduction, oxidation, reactions due to Hydrogen, and other reactions (Cannizzaro reaction, electrophilic substitution reaction)
- 12.13 Uses of aldehydes and ketones
Carboxylic Acids
- 12.14 Nomenclature and structure of carboxyl group
- 12.15 Methods of preparation of carboxylic acids- (1)from primary alcohols and aldehydes (2) from alkylbenzenes(3)from nitriles and amides (4)from Grignard reagents (5) from acyl halides and anhydrides (6) from esters
- 12.16 Physical properties
- 12.17 Chemical reactions: (i) Reactions involving cleavage of O-H bond-acidity, reactions with metals and alkalies (ii) Reactions involving cleavage of C-OH bond-formation of anhydride, reactions with PCl5, PCl3, SOCl2, esterification, and reaction with ammonia (iii) Reactions involving -COOH group-reduction, decarboxylation (iv) Substitution reactions in the hydrocarbon part – halogenation and ring substitution
- 12.18 Uses of carboxylic acids
Chapter 13 Organic Compounds Containing Nitrogen
I. Amines
- 13.1 Structure of amines
- 13.2 Classification
- 13.3 Nomenclature
- 13.4 Preparation of amines: reduction of nitro compounds, ammonolysis of alkyl halides, reduction of nitriles, reduction of amides, Gabriel phthalimide synthesis, and Hoffmann bromamide degradation reaction
- 13.5 Physical properties
- 13.6 Chemical reactions: the basic character of amines, alkylation, acylation, carbonyl amine reaction, reaction with nitrous acid, reaction with aryl sulphonyl chloride, the electrophilic substitution of aromatic amines-bromination, nitration and sulphonation
II. DIazonium Salts
- 13.7 Methods of preparation of diazonium salts (by diazotization)
- 13.8 Physical properties
- 13.9 Chemical reactions: Reactions involving
III. Cyanides and Isocyanides
- 13.10 Structure and nomenclature of cyanides and isocyanides Preparation, physical properties, and chemical reactions of cyanides and isocyanides
We hope that this Telangana & Andhra Pradesh BIEAP TS AP Intermediate Inter 2nd Year Chemistry Study Material Textbook Solutions Guide PDF Free Download 2022-2023 in English Medium and Telugu Medium helps the student to come out successful with flying colors in this examination. This Sr Inter 2nd Year Chemistry Study Material will help students to gain the right knowledge to tackle any type of questions that can be asked during the exams.