Students can go through AP State Board 10th Class Physical Science Notes Chapter 2 Acids, Bases and Salts to understand and remember the concept easily.
AP State Board Syllabus 10th Class Physical Science Notes Chapter 2 Acids, Bases and Salts
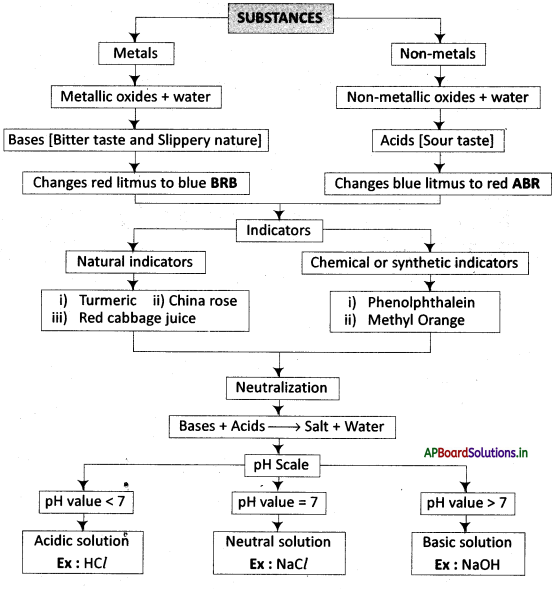
→ Any substance that has a sour taste and turns blue litmus to red is called an ‘acid’.
→ Any substance that has a bitter taste and soapy in nature that turns red litmus to blue is called a base.
→ We use natural and chemical indicators to test for acids and bases.
→ Acid-base indicators are dyes or mixtures of dyes that are used to indicate the presence of acids and bases.
→ When acids and bases react with each other, their respective salts and water are formed. This chemical reaction is called neutralization.
→ The acidic nature of a substance is due to the formation of H+ (aq) ions in the solution. The formation of OH– (aq) ions in the solution are responsible for the basic nature of a substance.
→ Some substances whose odour changes in acidic or basic media are called Olfactory indicators.
→ When a base reacts with a metal with the evolution of hydrogen gas, a salt is formed.
→ When an acid reacts with a metal carbonate or metal hydrogen carbonate gives the corresponding salt, carbon dioxide gas and water.
→ Metallic oxides react with acids to give salt and water. Metallic oxides are basic in nature.
→ Non-metallic oxides are acidic in nature.
→ Acidic and basic solutions in water conduct electricity because they produce hydrogen ions and hydroxide ions respectively.
→ Acid solutions have ions and the electric current is carried through the movement of ions in the solution.
→ In glucose and alcohol ions are absent.
→ Dissolving bases in water produces hydroxide (OH–) ions.
![]()
→ Bases that are soluble in water are called alkalis.
→ The process of dissolving an acid or a base in water is an exothermic process.
→ Mixing an acid or base with water results in dilution.
→ Strong acids are completely ionized whereas weak acids are partially Ionized.
→ Strong bases release more OH– ions in water, whereas weak bases release fewer OH– ions in water.
→ The strength of an acid or an alkali can be tested by using a scale called the pH scale (0-14) which gives the measure of hydrogen ion concentration in a solution.
→ A neutral solution has a pH of 7, while an acidic solution has a pH less than 7 and a basic solution has a pH of more than 7.
→ A mixture of several indicators is called a Universal indicator.
→ pH scale ¡s used for measuring hydrogen ion concentration in a solution.
→ Living beings carry out their metabolic activities within an optimal pH range.
→ Our body works within the pH range of 7.0 to 7.8.
→ When the pH of rainwater is less than 5.6, it is called acid rain.
→ Mixing concentrated acids or bases with water is a highly exothermic process.
→ Acids and bases neutralize each other to form corresponding salts and water.
→ The water of crystallization is the fixed number of water molecules chemically attached to each formula unit of salt in its crystalline form.
→ Antacids are the mild bases used to control the stomach pain caused due to indigestion.
→ Magnesium hydroxide is known as the ‘milk of magnesia’.
→ Tooth decay starts when the H of the mouth is lower than 5.5.
→ The atmosphere of Venus is made ¿p of thick white and yellowish clouds of sulphuric acid.
→ Salts are electrically neutral.
→ Beds of rock salts are formed when seas of bygone ages dried up.
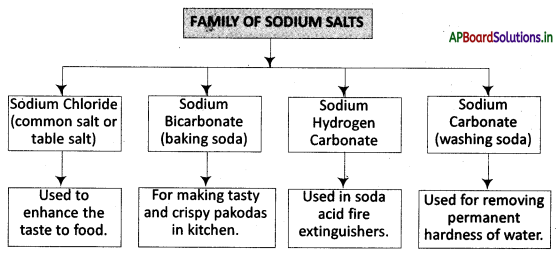
→ Sodium hydrogen carbonate is used in soda acid fire extinguishers.
→ Washing soda is used in the manufacture of borax.
→ Salts have various uses in everyday life and in industries.
→ Plaster of Paris (CaSO4, 6H20) is used for making toys, materials for decoration and for making surfaces smooth.
→ Indicator: A special substance that gives different colours in acidic, and basic media.
![]()
→ Acid: ‘Acid’ is a word derived from the Latin word ‘Acidus’. It means sour. Usually, we call any substance that has a sour taste.
→ Base: The substance that has a bitter taste and is slippery to touch.
→ Red Litmus: A red coloured indicator used to test the presence of basic nature in a substance.
→ Blue Litmus: A blue coloured indicator used to test the presence of acidic nature in a substance.
→ Phenolphthalein and Methyl Orange: The synthetic (chemical) indicators to find the presence of acids and bases.
→ Salts: A substance that is formed as a result of the neutralization reaction between an acid and a base.
→ Neutralization: When acids and bases react, salt and water are formed. This chemical reaction is known as neutralization.
→ Guard tube: A drying tube used in experiments.
→ Hydronium ion: H3O+ ion.
→ Alkali: A base that is soluble in water is called alkali.
→ Strong acid: Acid that gives more H30+ ions (They are ionized completely).
![]()
→ Strong Base: Base that gives more OH– ions in water.
→ Universal indicator: It is a mixture of several indicators.
→ pH Scale: A scale for measuring hydrogen ion concentration in a solution. pH = – log10 (H+)
→ Potenz: A scale for measuring hydrogen ion concentration in a solution is called the pH scale. The ‘P’s in pH stands for Potenz. In German ‘Potenz’ is power.
→ Antacids: A mild base used to get rid of pain and irritation caused due to indigestion of food.
→ Tooth decay: Corrosion of tooth due to the degradation of sugar and food particles remaining in the mouth.
→ Family of Salts: Salts having the same positive or negative radicals belong to a family called a family of salts.
→ Common Salt or Table Salt: Sodium Chloride Salt (NaCl).
→ Bleaching Powder: The action of chlorine on dry slaked lime.
→ Baking Soda: Sodium bicarbonate salt is used in the kitchen for making tasty and crispy pakoras.
→ Washing Soda: Sodium carbonate salt is used in washing clothes, etc.
→ Hydrated Salt: Salt which contains water.
→ The water of crystallization: It is the fixed number of water molecules chemically attached to each formula unit of salt in its crystalline form.
→ Plaster of Paris: On heating gypsum at 373 K it loses water molecules and becomes Calcium Sulphate hemihydrate called Plaster of Paris.
→ Olfactory indicators: The substances whose odour changes in acidic or basic media.
→ Litmus solution: A natural indicator extracted from certain lichens.
→ Lichen: Rock Moss.
→ Endothermic reaction: A chemical reaction in which heat is absorbed.
→ Exothermic reaction: A chemical reaction in which heat is evolved.
→ Aqueous Solution: The solution in which the solvent is water.
→ The concentration of a Solution: The amount of solute present per unit volume or per unit mass of the solution.
→ Weak acids: Acids that give fewer (less) H3O+ ions (They are not ionized completely).
→ Weak bases: Bases that give fewer (less) OH– ions in water.
![]()
→ Saturated solution: The solution in which the amount of solute dissolved is equal to solubility.
→ Unsaturated solution: The solution in which solute is less than the solvent.
→ Supersaturated solutIon: The solution in which the solute dissolved is more than its solubility.
→ Acid rain: When the pH of rainwater is less than 5.6, it is called acid rain.
→ Rock Salt: Deposits of seawater (solid salt) are changed to large brown crystals called Rock salt.
→ Dry slaked lime: Ca (OH)2 (i.e.,) Calcium Hydroxide.
→ Brine solution: An aqueous solution of common salt.
→ Anode: A positive electrode.
→ Cathode: A negative electrode.
→ Quick lime: CaO (Calcium Oxide).
![]()
→ Achara Nagarjuna (AD 931):
- Acharya Nagarjuna was born In AD 931 in Gujarat (India). He was an alchemist.
- He knew an artel transmuting base metals to look like gold.
- He was a metallurgist and chemist. He was very famous for his book Rasevada’ Which deals with Mercury compounds.
- He discussed the extraction of costly Metairie gold, alive; etc. In bis wrItings.
→ JFW Adolf Von Baeyer (1835 – 1917):
- Johann Friedrich Wilhelm Adoli Von Baeyer was born on October 31, 1835 In Berlin (Germany).
- He was interested In chemical experiments. He found a new double salt of copper.
- He studied Methyl Chloride, Uric acid, Indigo, etc.
- He discovered Indole.
- His ‘Baeyer strain theory of the Carbon iingW Is very famous.
- He received the Nobel Prize In 1905.