Students can go through AP State Board 8th Class Physical Science Notes Chapter 3 Matter Around Us to understand and remember the concept easily.
AP State Board Syllabus 8th Class Physical Science Notes Chapter 3 Matter Around Us
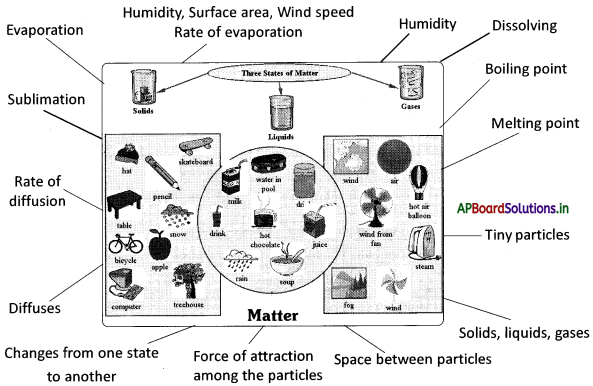
→ The matter is made up of tiny particles, which are small beyond our imagination.
→ Anything in this world that occupies space and has mass is considered as matter.
→ The matter is made up of tiny particles, which are small beyond our imagination
→ Matter exists in these states i.e. solids, liquids and gases.
→ Solids, liquids and gases differ in their properties.
→ Particles of matter move continuously in liquids and gases, known as diffusion.
→ Particles of matter have space between them.
→ The rate of diffusion of gases is higher than that of liquids and solids.
→ Dissolving is a process of occupying the space between liquid particles by solid particles, on the addition of a solid to a liquid.
![]()
→ Particles of matter have forces acting between them that keeps the particles together.
→ The force of attraction between the particles is maximum in solids, intermediate in liquids and minimum in gases.
→ The particles are arranged orderly in the case of solids, while particles move randomly in gases.
→ The states of matter can be changed by changing the temperature or pressure.
→ The temperature at which solid melts to become a liquid is called the ‘melting point’.
→ The process of changing solid to liquid is called ‘fusion’.
→ The amount of heat energy that is required to overcome the attraction energy among the particles is given by the latent heat of a substance.
→ The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
→ The phenomenon of change of a liquid into vapours at any temperature below its boiling point is called evaporation.
→ The rate of evaporation depends on the surface area, humidity and wind speed
→ Humidity is the amount of water vapour present in the air.
→ Matter: Anything in this world that occur íes space and has mass is considered as matter Ex: Water, food, air, tables, chairs, etc
→ States of matter: Matter exists in three states. They are solids, liquids and gases. These three differ in their properties.
→ Solid: Solids have a definite shape and ci fixed volume.
![]()
→ Liquid: Liquids can get their shape depending on the container. Liquids can flow easily from one container to another. Hence they are called fluids Ex: Water, milk, etc.
→ Gas: Gases neither have a fixed shape nor volume. Ex: Air, CNG, etc.
→ Particles: Matter is made up of tiny particles. Particle ¡s the smallest part of the matter.
→ Diffusion: The particles of matter move continuously in liquids and gases. This movement ¡s called diffusioni1
→ Compressibility: Large volume of gas ¡s compressed into cylinders of the small volume to make it portable. This property is called compressibility. (or) The reciprocal of the bulk modulus of the material.
→ Forces of attraction: The force of attraction between the particles is maximum in solids, intermediate in liquids and minimum in gases.
→ Evaporation: The phenomenon of change of a liquid into vapours at any temperature below its boiling point ¡s called evaporation.
→ Compressed Natural Gas: Natural gas at high volume, compressed into small cylinders, (CNG) so that they can be used in automobiles, etc.
→ Melting point: The temperature at which solid melts to become a liquid is called the melting point.
→ Fusion: The process of melting is called íusion.
→ Boiling point: The temperature at which a liquid starts boiling at the atmospheric pressure is known as its boiling point.
→ Sublimation: Some substances may change from solid to a gaseous state and vice versa without passing through the liquid state. This is called sublimation. Ex: Iodine, Camphor, etc.
→ Latent heat of fusion: The amount of heat energy that is required to overcome the attraction is given by the latent heat of the substance.
→ Space between particles: Particles of matter has space between them. This space is very high in gases, intermediate in liquids, very low ¡n solids.
→ Dissolving: Solid particles enter into the space between the liquid particles when we add a solid into a liquid. This is called dissolving. Ex: Addition of sugar to water.
→ Thermometer: A thermometer is a device used to measure the temperature of a substance, our body, etc.
![]()
→ Humidity: The amount of water vapour present in the air is called humidity.
→ Albert Einstein:
- Albert Einstein was born in 1879 in Germany.
- He is known throughout the world as a man who propounded the famous theory of relativity.
- Einstein was awarded the Nobel Prize in 1921.