Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material 4th Lesson Surface Chemistry Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material 4th Lesson Surface Chemistry
Very Short Answer Questions
Question 1.
What is an interface? Give one example.
Answer:
The interface is normally a few molecules thick but its area depends on the size of the particles of bulk.
Interface is represented by separating the bulk phases by hyphen (-) (or) a slash (/).
Eg : Interface between a solid and a gas may be represented by solid-gas (or) solid/gas.
Question 2.
What is adsorption ? Give one example.
Answer:
Adsorption : The accumulation (or) concentration of a substance on the surface rather than in the bulk of solid (or) liquid is known as adsorption.
Eg : Adsorption of gases like O2, H2, Cl2 etc., on charcoal.
![]()
Question 3.
What is absorption ? Give one example.
Answer:
Absorption : The uniform distribution of a substance through out the bulk of the solid substance is known as absorption.
Eg : Chalk stick dipped in ink.
Question 4.
Distinguish between adsorption and absorption. Give one example of each.
Answer:
Adsorption
1. The accumulation (or) concentration of a substance on the surface rather than in the bulk of solid (or) liquid is known as adsorption.
Eg :Adsorption of gases like O2, H2, Cl2 etc., on charcoal.
Absorption
1) The uniform distribution of a susbstance through out the bulk of the solid substance is known as absorption.
Eg : Chalk stick dipped in ink.
Question 5.
The moist air becomes dry in the presence of silica gel. Give reason for this.
Answer: The moist air becomes dry in presence of silica gel because the water molecules present in air get adsorbed on the surface of the gel.
Question 6.
Methylene blue solution when shakes with animal charcoal gives a colourless filtrate on filteration. Give the reason.
Answer:
Methylene blue solution (organic dye) when shaken with animal charcoal gives a colourless filtrate on filtration. The molecules of the dye from the solution are adsorbed on the charcoal.
![]()
Question 7.
A small amount of silica gel and a small amount of anhydrous calcium chloride are placed separately in two corners of a vessel containing water vapour. What phenomena will occur ?
Answer:
A small amount of silica gel and a small amount of anhydrous CaCl2 are placed separately in two comers of a vessel of water vapour. Water vapours are is absorbed by anhydrous CaCl2 but adsorbed by silical gel.
Question 8.
What is desorption ?
Answer:
Desorption: The process of removing aa adsorbed substance from a surface on which it is adsorbed is called desorption.
Question 9.
What is sorption ?
Answer:
In case of some substances both adsorption and absorption takes place. This phenomenon is called sorption.
Question 10.
Amongst adsorption, absorption which is a surface phenomena and why ?
Answer:
- Adsorption is a surface phenomenon.
- In adsorption the concentration of the adsorbate increases only at the surface of the adsorbent. While in absorption the concentration is uniformly distributed throughout the bulk of the compound.
Question 11.
What is the name given to the phenomenon when both absorption and adsorption take place together ?
Answer:
The name of the phenomenon when both adsorption and absorption take place together is sorption.
![]()
Question 12.
Chalk stick dipped in an ink solution exhibits the following :
a) The surface of the stick retains the colour of the ink.
b) Breaking the chalk stick,-it is found still white from inside.Explain the above observations.
Answer:
a) Chalk stick dipped in an ink solution, the surface of the stick retains the colour of the ink due to adsorption of coloured molecules in the ink.
b) Chalk stick dipped in an ink solution, the surface of the stick retains the colour of the ink due to adsorption of coloured molecules in the ink while the solvent of the in k goes deep into the stick due to absorption so on breaking the chalk stick, it is found to be white inside.
Question 13.
What are the factors which influence the adsorption of a gas on a solid ?
Answer:
Adsorption of a gas on solids is influenced by the following factors.
a) Surface area of the adsorbent, b) Nature of the gas, c) Pressure of the adsorbate, d) Temperature.
Question 14.
Why is adsorption always exothermic ?
Answer:
During the adsorption, there is always a decrease in residual forces of the surface. There is decrease in surface energy which appears as heat.
∴ Adsorption is always Exothermic.
Question 15.
Give the signs of ∆H & ∆S, when ammonia gas gets adsorbed on charcoal.
Answer:
When NH3 gas gets adsorbed on charcoal the sign of ∆H is negative and the sign of ∆S is also negative.
Question 16.
How many types of adsorption are known ? What are they ?
Answer:
Adsorption process is divided into two types.
1) Physisorption 2) Chemisorption
Question 17.
What types of forces are involved in physisorption of a gas on solid ?
Answer:
Weak Vander waals forces are involved in physisorption of a gas on solid.
![]()
Question 18.
What type of interaction occurring between gas molecules and a solid surface is responsible for chemisorption of the gas on solid.
Answer:
Chemical bonds occur between gas molecules and a solid surface, is responsible for chemisorption of the gas on solid.
Question 19.
Why chemisorption is called activated adsorption ?
Answer:
Chemisorption involves a high energy of activation. Hence it is referred as activated adsorption.
Question 20.
What is the difference between physisorption and chemisorption ?
Answer:
1) In physisorption the forces present between adsorbate and adsorbent are weak vander . waal’s forces.
2) In chemisorption the forces present between adsorbate and adsobent are chemical bonds (valency forces).
Question 21.
Out of physisorption and chemisorption, which can be reversed ?
Answer:
Out of physisorption and chemisorption, phyisorption is reversed (reversible).
Question 22.
How is adsorption of a gas related to its critical temperature ?
Answer:
Higher the critical temperature, greater is the case of liquefaction of a gas. Then the extent of adsorption will be high.
Question 23.
The critical temperature of SO2 is 630 K and that of CH4 is 190 K. Which is adsorbed easily on activated charcoal ? Why ?
Answer:
Given the critical temperature of SO2 is 630 K and that of CH4 is 190 K.
SO2 gas is adsorbed easily on activated charcoal because of higher critical temperature. Higher the critical temperature of gas, extent of adsorption is high.
Question 24.
Easily liquefiable gases aye readily adsorbed on solids. Why ?
Answer:
Easily liquefiable gases are readily adsorbed on solids because vander waal’s forces are stronger near the critical temperatures.
![]()
Question 25.
Amongst SO2, H2 which will be adsorbed more readily on the surface of charcoal and why ?
Answer:
Among SO2, H2 gases, SO2 gas adsorb more readily on the surface of charcoal because SO2 has high critical temperature (630 K) than dihydrogen (33K).
Question 26.
Compare the enthalpy of adsorption for physisorption and chemisorption.
Answer:
- In physical adsorption enthalpy of adsorption is low (20 – 40 KJ/mole)
- In case of chemical adsorption enthalpy of adsorption is high (80 – 240 KJ/mole)
Question 27.
What is the magnitude of enthalpy of physical adsorption ? Give reason for this magnitude.
Answer:
The magnitude of enthalpy of physical adsorption is low 20 – 40 KJ ,/mole. This is due to the presence of weak vander waal’s forces between gas molecules and solid surface.
Question 28.
What is the magnitude of enthalpy of chemisorption ? Give reason for this magnitude.
Answer:
The magnitude of enthalpy of chemical adsorption is high 80 – 240 KJ /mole. This is due to the presence of chemical bonds between gas molecules and solid surface.
Question 29.
Give any two applications of adsorption.
Answer:
Applications of adsorption :
a) Separation of inert gases : Different noble gases adsorb at different temperatures on coconut charcoal. By this principle (adsorption) mixture of noble gas is separated by adsorption on coconut charcoal.
b) Gas masks : Gas mask is a device which consists of activated charcoal (or) mixture of adsorbents is used by coal miners to adsorb poisonous gases during breathing.
Question 30.
Why physisorption suffers from lack of specificity ?
Answer:
Physisorption suffers from lack of specificity.
Explanation: A given surface of an adsorbent doesnot show any preference of a particular gas as the vander waal’s forces are universal.
Question 31.
What is an adsorption isotherm ? Write the equation of Freundlich adsorption isotherm.
Answer:
Adsorption Isotherm : The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve known as adsorption isotherm.
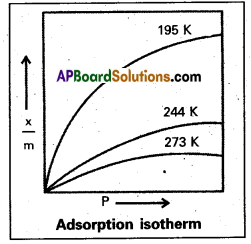
Freundlich adsorption isotherm equation is \(\frac{x}{m}\) = k. P1/n
x = mass of the gas adsorbed
m = mass of the adsorbent
P, k and n are constants.
![]()
Question 32.
In the Freundlich adsorption isotherm, mention the conditions under which, following graph will be true ?
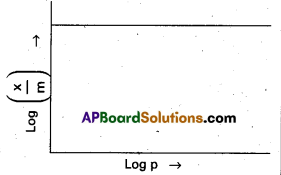
Answer:
In the above graph extent of adsorption does not depends on the pressure.
When \(\frac{1}{n}\) = 0, \(\frac{x}{m}\) = constant, the adsorption is independent of pressure.
Question 33.
What role does adsorption play in heterogenous catalysis ?
Answer:
In heterogeneous catalysis, adsorption of reactants on the solid surface of the catalysts increases the rate of reaction.
Eg : Manufacturing of NH3 using Fe-as catalyst [Haber’s process].
Question 34.
What is the role of MnO2 in the preparation of O2 from KClO3 ?
Ans. The chemical equation for the preparation of O2 from KClO3 is
![]()
MnO2 increases the rate of reaction. Hence it is a catalyst for the above reaction.
Question 35.
Define “promoters” and “poisons” in the phenamenon of catalysis ?
Answer:
Promoters : The substances with enhance the activity of catalyst are known as promoters. Poisons : The substances which decrease the activity of a catalyst are known as poisons.
Question 36.
What is homogeneous catalysis ? How is it different from heterogeneous catalysis ?
Answer:
Homogeneous Catalysis : The catalysis in which reactants and catalyst are in same phase is called Homogeneous catalysis.
In case of heterogeneous catalysis, catalyst and reactants are present in different phases where as in case of homogeneous catalysis catalyst and reactants are present in same phase.
Question 37.
Give two examples for homogeneous catalytic reactions.
Answer:
The following are the examples for homogeneous catalytic reactions.
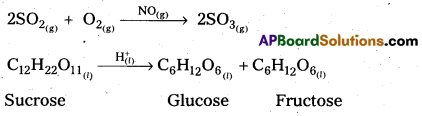
Question 38.
Give two examples for heterogeneous catalysis.
Answer:
The following are the examples of heterogeneous catalytic reactions.
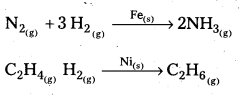
![]()
Question 39.
Give two examples which indicate the selectivity of heterogeneous catalysis.
Answer:
The following reactions indicate the selectivity of heterogeneous catalysis.
Starting with H2 and CO, and using different catalysts, we get different products.
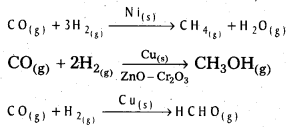
The action of a catalyst is highly selective in nature. A substance which acts as a catalyst in one reaction may fail to catalyse another reaction.
Question 40.
Why zeolites are treated as shape selective catalysts ?
Answer:
- The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape selective catalysts.
- Zeolites are shope selective catalysts because of their honey comb-like structure. They are microporous alumino silicates with 3-dimensional net work of silicates.
Question 41.
Which zeolite catalyst is used to convert alcohols directly into gasoline ?
Answer:
Zeolite ZSM – 5 is used to convert alcohols directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
Question 42.
What are enzymes ? What is their role in human body ?
Answer:
Enzymes are complex nitrogenous organic compounds which are produced by living plants and animals.
These act as specific catalysts in biological reactions.
These catalyse the numerous reactions that occur in the bodies of animals and plants to maintain the life process.
Question 43.
Can catalyst increase the yield of reaction ?
Answer:
Catalyst does not increase the yield of reaction. Catalyst just speed up the product formation.
Question 44.
Name any two enzyme catalyzed reactions. Give the reactions.
Answer:
1) Inversion of Cane Sugar :

2) Decomposition of urea into ammonia and CO2:
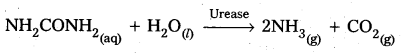
Question 45.
Name the enzymes obtained from soyabean source.
Answer:
The enzyme obtained from soyabean source is urease.
![]()
Question 46.
Name the enzymes used in
a) Decomposition of urea into ammonia.
b) Conversion of proteins into peptides in stomach.
Answer:
a) The enzyme used in the decomposition of urea into ammonia is urease.
b) The enzyme used in conversion of proteins into peptides in stomach is pepsin.
Question 47.
What enzymes are obtained from yeast ?
Answer:
The enzymes obtained from yeast are invertase, zymase and maltase.
Question 48.
At what ranges of temperature and pH, enzymes are active ?
Answer:
The optimum temperature range for enzymatic activity is 298 – 310 K. Human body temperature being 310 K is suited for enzyme catalysed reactions. The rate of an enzyme – catalysed reaction is maximum at a particular pH called optimum pH, which lies between 5-7.
Question 49.
Represent diagrammatically the mechanism of the enzyme catalyis.
Answer:
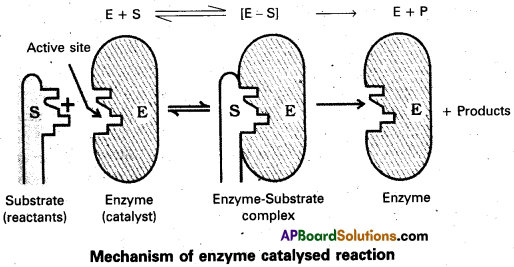
Question 50.
Name any two industrially important heterogeneous catalytic reactions mentioning the catalysts used.
Answer:
i) Manufacturing of NH3 by Haber’s process
![]()
ii) Hydrogenation of vegetable oils in presence of finely divided nickel as catalyst.
![]()
The above mentioned are industrially important heterogeneous catalytic reactions.
Question 51.
What is a colloidal solution ? How is it different from a true solution with respect to dispersed particle size and homogeneity ?
Answer:
A heterogeneous system in which one substance is dispersed as large particles in another substance is called colloidal solution.
- In colloidal solution the particle size of the dispersed phase is of the order of 1 mμ – 1 μ where as in true solution the particle size of the solute are the order of mp or less.
- Colloidal solution is a heterogeneous binary system where as true solution is a homogeneous binary system.
![]()
Question 52.
Name the dispersed phase and a dispersion medium in the following colloidal systems
- fog
- smoke
- milk.
Answer:
- Fog : Dispersed phase → liquid
Dispersion medium → gas - Smoke : Dispersed phase → carbon particles (solid)
Dispersion medium → Air - Milk : Dispersed phase → liquid fat
Dispersion medium → water
Question 53.
What are lyophilic and lyophobic sols ? Give one example for each type.
Answer:
Lyophilic colloid : The colloidal solution in which the dispersed phase has great affinity to the dispersion medium is called a Lyophilic colloid or Lyophilic solution.
Ex : Starch solution.
The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid : The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution.
Ex: Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution.
Gold particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
Question 54.
Explain the terms with suitable examples .
- aerosol
- hydrosol.
Answer:
- Aerosol: The colloidal solution in which dispersed phase is solid and dispersion medium is gas is called an aerosol.
Eg : Smoke, dust. - Hydrosol: In a colloidal solution dispersion medium is water them it is called aquasol (or) hydrosol.
Eg : Milk, Gold sol.
Question 55.
Explain why lyophilic colloids are relatively more stable than lyophobic colloids ?
Answer:
- Lyophilic colloids are reversible and are quite stable. These are not coagulated.
- Lyophobic sols are irreversible and need stabilizing agents for their stabilization. These sols are readily precipitated on the addition of small amounts of electrolytes.
Question 56.
Give two examples of colloidal solutions of liquids dispersed in solid. What is the name given to the colloidal solution ?
Answer:
Cheese, butter, jellies are examples of colloidal solutions of liquids dispersed in solid. The name of the colloidal solution given to this type is Gels.
![]()
Question 57.
What is the difference between multimolecular and macromolecular colloids ? Give one example for each.
Answer:
- In multimolecular colloids, a large number of atoms (or) small molecules of the dispersed phase aggregate together to form species in the colloidal range.
- Macro molecules in suitable solvents form solutions in which the sizes of the macromolecules are in the colloidal range.
Question 58.
What are micelles ? Give one example.
Answer:
Micelles : Some substances which at low concentrations behave as normal strong electrolytes, but at high concentrations exhibit colloidal behaviour due to formation of aggregates. The aggregated particles thus formed are called micelles.
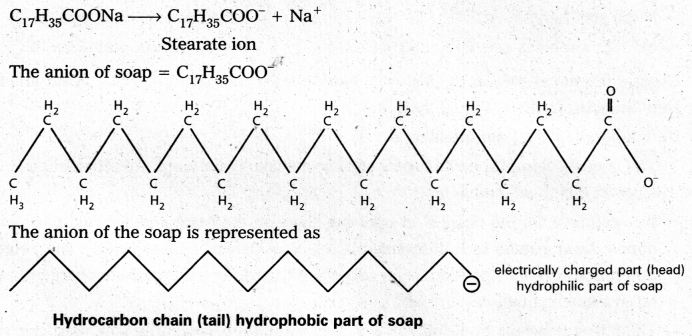
Eg : Stearate ions (C17H35COO–) associate together in high concentration, in a solution of soap in water and forms a micelle.
Question 59.
How do micelles differ from a normal colloidal solutions ?
Answer:
Some substances which at low concentrations behave as normal strong electrolytes, but at high concentrations exhibit colloidal behaviour due to formation of aggregates. The aggregated particles thus formed are called micelles.
- hese are also known as associated colloids.
- These colloids have both lyophilic and lyophobic parts.
- Micelles may contain as many as 100 or more of normal molecules.
- On dilution these colloids revert back to individual electrolytes.
Question 60.
Give two examples of associated colloids.
Answer:
Surface active agents such as soaps and synthetic detergents are examples of associated colloids.
Question 61.
Can the same substance act both as colloid and crystalloid ?
Answer:
Yes, Micelles, (associated colloids) act as normal strong electrolytes at low concentrations and exhibit colloid behavior at high concentrations.
![]()
Question 62.
Give two examples of lyophobic sols.
Answer:
Metal sols and metal suiphide sols are examples of lyophobic colloids
- Gold sol is lyophobic colloid
Question 63.
Give examples of’colloidal system of
- Liquid In solid
- Gas in solid.
Answer:
- Cheese, butter, jellies are examples of liquid in solid type colloidal system.
- Foam rubber, pumice ‘stone are examples of gas in solid type of colloidal system.
Question 64.
What type of substances form lyophobic sols?
Answer:
Substances like metals, their sulphides.donot form colloidal sol simply by mixing with the dispersion medium. These form colloids by special methods, These sols are lyophobic colloids.
Question 65.
What is critical micelle concentration (CMC) and kraft temperature (Tk) ?
Answer:
The formation of micelles takes place only above a particular temperature called Kraft temperature (Tk) and above a particular concentration called Critical micelle concentration (CMC).
Question 66.
Why lyophobic colloids are called irreversible colloids ?
Answer:
- Lyophobic colloids are coagulated by the addition of small amounts of electrolytes.
- The precipitates does not give back the colloidal sol by same addition of the dispersion medium.
- Hence lyophobic sols are called irreversible sols.
Question 67.
How a colloidal sol of arsenous sulphide is prepared ?
Answer:
Colloidal sol of arsenous sulphide is prepared by the double decompostion of As2O3 and H2S as follows.
![]()
![]()
Question 68.
What is Peptization ?
Answer:
Peptization : The process of converting a precipitate into colloidal sol by shaking it with the dispersion medium in the presence of a small amount of electrolyte is called Peptization.
Question 69.
What is dialysis ? How is dialysis can be made fast ?
Answer:
Dialysis : The process of removing a dissolved substance from a colloidal solution using a suitable membrane is called dialysis.
Dialysis is made faster by applying an Emf. This is known as Electrodialysis.
Question 70.
What is collodion solution ?
Answer:
Collodion solution : Collodion is a 4% solution of nitro cellulose in a mixture of alcohol and ether.
Question 71.
How an ultrafilter paper is prepared from ordinary filter paper ?
Answer:
Ultra filter paper is prepared by soaking the filter paper in collodion solution, hardening by formaldehyde and then finally dried.
Question 72.
What is Tyndall effect ?
Answer:
Tyndall effect : When light enters a colloidal solution, it is scattered by the large sized colloidal dispersed phase particles. Therefore when light passes through a solution we will be able to see the path of the light as a luminous beam. This is called Tyndall effect.
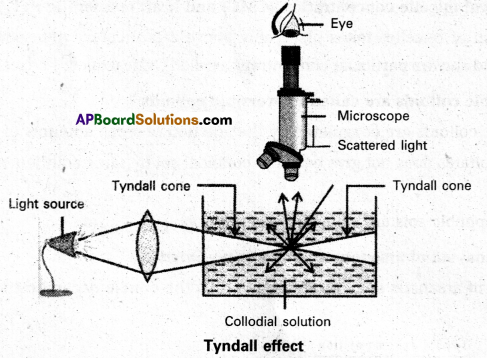
This is an optical property exhibited by colloidal solution. This phenomenon is clearly seen with a microscope placed at right angles to the path of light. Colloidal particles become self luminous due to absorption of light. A true solution does not show Tyndall effect.
Question 73.
What conditions is tyndall effect observed ?
Answer:
Tyndall effect is observed only when
- The diameter of the dispersed particles is not much smaller than the wave length of the light used.
- The refractive indices of the dispersed phase and dispersion medium differ greatly in magnitude.
![]()
Question 74.
Can Tyndall effect be used to distinguish between a colloidal solution and a true solution ? Explain.
Answer:
Tyndall effect is used to distinguish between a colloidal solution and a true solution.
- True solution placed in dark and is observed in the direction of the light passed beam passing through it. It appears clear and it is observed from a direction at right angles to the direction of the light beam, it appears perfectly dark.
- Colloidal solution viewed in the same way may appear reasonably clear in the direction of light but they show a mild to strong opalescence when viewed at right angles to the direction of light.
Question 75.
Sky appears blue in colour. Explain.
Answer:
Dust particles along with water vapour suspended in air scatter blue light which reaches our eyes and hence the sky looks blue to us.
Question 76.
What is Brownian movement.
Answer:
Brownian movement: This is a kinetic property of colloidal solution.
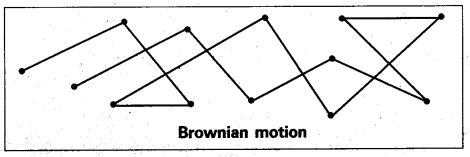
When a colloidal solution is examined by ultramicroscope, the colloidal particles are seem to be moving in a rapid zig-zag motion.
This rapid motion of colloidal particles is called Brownian movement.
This motion is due to unequal bombardment of colloidal particles by molecules of dispersion medium. Smaller the colloidal particles the more rapid is the Brownian movement.
Question 77.
What is the main cause for charge on a colloidal solution ?
Answer:
The charge on the colloidal particles is due to electron capture by sol particles during electro dispersion of metals , and due to preferential adsorption ions from solution (or) due to formulation of electrical double layer.
Question 78.
What is electrokinetic potential or zeta potential ?
Answer:
In a colloidal sol the charges of opposite signs on the fixed and diffused parts of the double layer results in a difference in potential between these layers. The potential difference between the fixed layer and the diffused layer of opposite charge is called electro kinetic potential (or) zeta potential.
![]()
Question 79.
Write the chemical formula of positively charged and negatively charged hydrated ferric oxide colloidal solutions.
Answer:
- Formula of positively charged hydrated ferric oxide is Fe2O3.xH2O/Fe+3
- Formula of negatively charged hydrated ferric oxide is Fe2O3.xH20/OH–
Question 80.
Give the order of coagulating power of Cl, SO42-, PO43- in the coagulation of positive sols.
Answer:
The order of coagulating ability’of given ions with positive sols is PO43- > SO42- > Cl–
Question 81.
Amongst Na+, Ba2+, Al3+, which coagulates negative sol readily and why ?
Answer:
The order of coagulating ability of given ions with negative sols is Al+3 > Ba+2 > Na+
Question 82.
A colloidal solution of AgI is positively charged when prepared from a solution containing excess of Ag+ ions and negatively charged when prepared from a solution containing excess of I– ions Explain.
Answer:
When a dilute AgNO3 solution is added to a dilute KI solution taken in excess, the precipitated Agl adsorbs I– ions present in excess and a negatively charged AgI colloidal solution is formed. However when dilute KI solution is added to dilute AgNO3 solution taken in excess, AgI precipitate adsorbs Ag+ ions present in excess and a positively charged AgI colloidal solution is formed. Generally the ion common to dispersed particle is adsorbed. .
AgI/I–
Negatively charged
AgI/Ag+
Positively charged.
Question 83.
What is electrophoresis ?
Answer:
When electric potential is applied across two platinum electrodes dipping in a colloidal solution,-the colloidal particles move towards one or the other electrode. The movement of colloidal particles under an applied emf is called “electrophoresis”.
Question 84.
What is electro osmosis ?
Answer:
If the movement of colloidal particles is arrested by some suitable means, the dispersion medium moves in opposite direction. This phenomenon is termed “electro osmosis”.
Question 85.
What is coagulation ?
Answer:
The stability of the lyophilic sols is due to the presence of charge on the colloidal particles. If this charge is neutralised the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity. This process of settling down of colloidal particles is called coagulation (or) flocculation (or) precipitation.
![]()
Question 86.
Define flocculation value.
Answer:
The minimum concentration of an electrolyte in millimoles per litre required to cause coagulation of a sol in two hours is called “coagulating value” (or) flocculation value.
Question 87.
State Hardy – Schulze rule.
Answer:
Greater the valence of the coagulating ion added, the greater is its power to cause coagulation. This is known as Hardy-Schulze rule.
Questions 88.
Coagulation takes place when sodium chloride solution is added to a colloidal solution of hydrated ferric oxide. Explain.
Answer:
When NaCl solution is added to the colloidal solution of hydrated ferric oxide coagulation takes place (precipitation formed). .
The reason is that colloidal particles interact with ions (Na+, Cl–) carrying charge opposite to that present on themselves. This causes neutralisation of charges leading to their coagulation.
Question 89.
How are lyophobic solutions protected from phenomenon of coagulation.
Answer:
Lyophobic sols are protected from the phenomenon of coagulation by adding suitable lyophilic sol.
Question 90.
What is protective colloid ?
Answer:
Lyophilic colloids used for the prevention of coagulation of lyophobic colloids are called protective colloids.
- Lyophilic colloids protect the lyophobic colloids.
Question 91.
What is an emulsion ? Give two examples. [A.P. Mar. 17]
Answer:
Emulsion: The colloidal system in which a dispersion of finely divided droplets of a liquid in another liquid medium is called emulsion.
Eg : Milk, Vanishing cream, Cold cream.
Question 92.
How emulsions are classified ? Give one example for each type of emulsion. [A.P. Mar. 17]
Answer:
Emulsion : A dispersion of finely divided droplets of a liquid in another liquid medium is called’emulsion’.
Ex: Milk.
In Milk, the droplets of liquid fat are dispersed in water. This is an example for oil in water type emulsion.
Classification of emulsions : Emulsions are “classified into two classes. These are
a) oil in water (O/W) and
b) water in oil (W/O), (O = Oil; W = Water).
These emulsions are classified as such depending on which is dispersed phase and which is dispersion medium.
a) Oil in Water (O/W) type emulsions : In this type of emulsions, the dispersed phase is oil and the dispersion medium is water.
Ex : Milk, liquid, fat (oil) in water.
Vanishing cream; fat in water.
b) Water in Oil (W/O) type emulsions : In this type of emulsions, the dispersed phase is water and the dispersion medium is oil.
Ex : Stiff greases : water in lubrication oils
Cod liver oil : water in cod liver oil
Cold cream : water in fat.
Question 93.
What is an emulsifying agent ?
Answer:
Emulsifying agent: The third substance which is added in small amounts to an emulsion to stabilize the emulsion is called emulsifying agent.
![]()
Question 94.
What is demulsification ? Name two demulsifiers.
Answer:
Demulsification : The separation of an emulsion into constituent liquids is known as demulsification.
Question 95.
How is artificial rain produced ?
Answer:
Artificial rain is produced by throwing electrified sand (or) spraying a sol carrying charge opposite to the one on clouds from an aeroplane.
Question 96.
Bleeding from fresh cut can be Stopped by applying alum. Give reasons.
Answer:
Bleeding from fresh cut can be stopped by applying alum. This is due to styptic action of alum which coagulates the blood and stops further bleeding.
Question 97.
Deltas are formed at the points where river enters the sea. Why ?
Answer:
Deltas are formed at the points where river enters the sea.
Explantation : River water is a colloidal solution of clay. Sea water contains a number of electrolytes. When river water meets the sea water, the electrolytes present in sea water coagulate the colloidal solution of clay resulting in its deposition of clay with the formation of delta.
Question 98.
Name any two applications of colloidal solutions.
Answer:
Applications of colloidal solutions :
Rubber: Plant latex is a colloidal solution of rubber particles which are negatively charged. Rubber is obtained from latex by coagulation.
Industrial Products : Paints, inks, synthetic plastics, rubber, graphite, lubricants, cement, etc., are all colloidal in nature.
Question 99.
How can aerial pollution by colloidal particles of smoke be prevented ? Explain.
Answer:
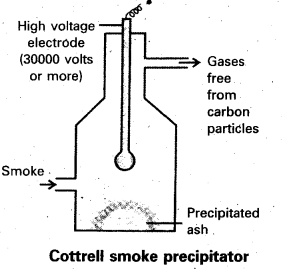
Aerial pollution by colloidal particles of smoke be prevented by electrical precipitation of smoke. Smoke is a colloidal solution of solid particles such as carbon, arsenic compounds, dust, etc., in air. The smoke, before it comes out from the chimney, is led through a precipitator containing plates having a charge opposite to that carried by smoke particles. The particles on coming in contact with these plates lose their charge and get precipitated. The particles thus settle down on the floor of the chamber. The precipitator is called cottrell precipitator.
Question 100.
Alum is used to purify water obtained from natural sources. Explain.
Answer:
The water obtained from natural sources often contains suspended impurities. Alum is added to such water to coagulate the suspended impurities and make water fit for drinking purposes.
![]()
Question 101.
Why medicines are more effective in colloidal state ?
Answer:
Colloidal medicines are more effective because they have large surface area and are therefore easily assimilated.
Question 102.
How rubber is obtained from latex ?
Answer:
Plant latex is a colloidal solution of rubber particles which are negatively charged. Rubber is obtained from latex by coagulation.
Question 103.
Name the type of emulsion to which milk belongs.
Answer:
Milk is a oil in water type of emulsion.
Dispersed phase : liquid fat.
Dispersion medium : water.
Short Answer Questions
Question 1.
What is adsorption ? Discuss the mechanism of adsorption of gases on solids.
Answer:
Adsorption : The accumulation (or) concentration of a substance on the surface rather than in the bulk of solid (or) liquid is known as adsorption.
Eg : Adsorption of gases like O2, H2, Cl2 etc., on charcoal.
Mechanism of Adsorption :
- Adsorption arises due to the fact that the surface particles of the adsorbent are not in the same environment as the particles inside the bulk.
- Adsorbent possess unbalanced or residual attractive forces. These forces are responsible for attracting the adsorbate molecules on adsorbent surface.
- The extent of adsorption increases with increase in surface area of adsorbent at a given temperature and pressure.
- During the adsorption, there is always a decrease in residual forces of the surface. There is decrease in surface energy which appears as heat. So, Adsorption is always Exothermic.
- Adsorption is accompanied by decrease in enthalpy as well as decrease in entropy of system. .
- As the adsorption proceeds AH becomes less and less negative, ultimately, AH becomes equal to TAS and AG becomes zero. At this state equilibrium is attained.
Question 2.
What are different types of adsorption ? Give any four differences between characteristics of these different types. [T.S. Mar. 19, 15; A.P. Mar. 15]
Answer:
Adsorption process is divided into two types.
1) Physisorption
2) Chemisorption.
Distinguishing characteristics of Physisorption and Chemisorption are given in the following table:
Physisorption
- This process is weak, due to vander Waal’s forces.
- The process is reversible.
- This is a quick process, i.e., takes place quickly.
- The process decreases with increase of temperature.
- This is a multilayered process.
- The process depends mainly on the nature of the adsorbent.
Chemisorption
- This process is strong, due to chemical forces.
- The process is irreversible.
- This is a slow process.
- The process increases with increase of temperature.
- This is a unilayered process.
- The process depends both on the nature of adsorbent and adsorbate.
![]()
Question 3.
What do you understand by the terms given below (a) absorption (b) Adsorption (c) Adsorbent and Adsorbate
Answer:
Absorption : The uniform distribution of a susbstance through out the bulk of the solid .substance is known as absorption. Eg: Chalk stick dipped in ink.
Adsorption : The accumulation (or) concentration of a substance on the surface rather than in the bulk of solid (or) liquid is known as adsorption.
Eg: Adsorption of gases like O2, H2, Cl2 etc., on charcoal.
Adsorbent: The substance on whose surface the adsorption occurs is known as adsorbent.
Adsorbate : The substance whose molecules get adsorbed on the surface of the adsorbent is known as adsorbate.
Eg: In the adsorption of acetic acid by charcoal, acetic acid is adsorbate and the charcoal is adsorbent.
Question 4.
Adsorption of a gas on the surface of solid is generally accompanied by decrease in entropy. Still it is a spontaneous process. Explain.
Answer:
Adsorption is accompanied by decrease in enthalpy as well as decrease in entropy of the system.
- For a process to be spontaneus there is a decrease in Gibbs Energy. .
- On the basis of equation ∆G = ∆H – T∆S; ∆G = -Ve
- If AH has sufficiently high negative value and T∆S is positive.
- In an adsorption process which ¡s spontaneous ∆G becomes negative by combining above.
- As the adsorption proceeds ∆H becomes less and less negative ultimately, ∆H becomes equal to T∆S and ∆G becomes zero. At this state equilibrium is attained.
Question 5.
How can the constants k and n of the Freundlich adsorption equation be calculated?
Answer:
Freundlich adsorption isotherm equation is
\(\frac{x}{m}\) = k. P =, x/m = Extent of adsorption ⇒ P = Pressure
k and n are constants which depend on the nature of the adsorbent and the gas at a particular temperature.
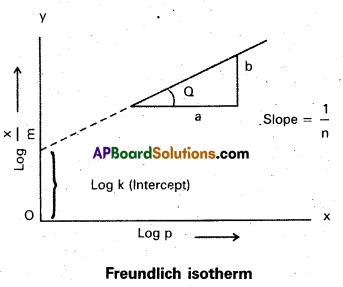
Applying logarithm to the above equation
log \(\frac{x}{m}\) = log k + \(\frac{1}{n}\) log P.
- A graph is plotted taking log \(\frac{x}{m}\) on y – axis and log P on x – axis. 1f the graph is a straight line then Freundlich isotherm is valid.
- The slope of the straight line gives \(\frac{1}{n}\) value.
- The intercept on the y-axis gives value of log k.
- \(\frac{1}{n}\) has values between 0 and 1
- When \(\frac{1}{n}\), = 0, \(\frac{x}{m}\) = constant, the adsorption is independent of pressure
\(\frac{1}{n}\) = 1, \(\frac{x}{m}\) = kP i.e., \(\frac{x}{m}\) ∝ p.
![]()
Question 6.
How does the extent of adsorption depend upon
a) Increasing the surface area per unit mass of adsorbent.
b) Increasing temperature of the system.
c) Increasing pressure of the gas.
Answer:
a) The extent of adsorption increases by increasing the surface area per unit mass of adsorbent.
b) The extent of adsorption decrease with an increase in temperature.
c) The extent of adsorption increase with increasing pressure of the gas.
Question 7.
What is catalysis ? How is catalysis classified ? Give two examples for each type of catalysis. [A.P. Mar. 16] [Mar. 14]
Answer:
Catalysis : A substance which alters the rate of a chemical reaction without itself being consumed in the process, is called a catalyst.
The action of catalyst in altering the rate of a chemical reaction is called catalysis.
Types of catalysis: Catalysis is classified into two types as
a) Homogeneous catalysis and b) Heterogeneous catalysis.
a) Homogeneous catalysis : The catalytic process in which the catalyst is present in the same phase as that of reactants, is known as homogeneous catalysis.
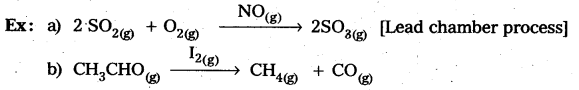
b) Heterogeneous catalysis : The catalytic process in which the catalyst is present in a phase different from that of the reactants is known as heterogeneous catalysis.
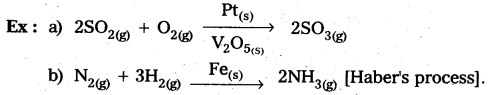
Question 8.
Discuss the mechanism involved in adsorption of heterogeneous catalysis.
Answer:
Adsorption theory of heterogeneous catalysis :
Adsorption theory explains the mechanism of heterogeneous catalysis.
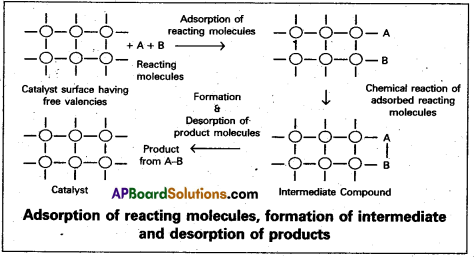
- The modern adsorption theory is the combination of the intermediate compound formation theory and the old adsorption theory.
- The mechanism of catalysis involves five steps.
- Diffusion of the reactants to the surface of the catalyst.
- Adsorption of the reactant molecules on the surface of the catalyst.
- Occurrence of chemical reaction on the catalyst’s surface through formation of an . intermediate.
- Desorption of reaction products from the catalyst surface, and thereby making the surface available again for more reaction to occur.
- Diffusion of reaction products away from the catalyst’s surface.
Question 9.
Discuss some features of catalysis by zeolites.
Answer:
The catalytic reaction that depends upon the pore structure of the catalyst and the size of the reactant and product molecules is called shape selective catalysts.
- Zeolites are shope selective catalysts because of their honey comb-like structure. They are microporous alumino silicates with 3-dimensional net work of silicates.
- Zeolites are microporous alumino silicates with dimensional network of silicates in which some Si-atoms are replaced by aluminium atoms giving Al-o-Si frame work.
- The reactions taking place in zeolites depend upon the size and shape of reactant and product molecules as well as on the pores and cavities of the zeolites.
- Zeolites are found in nature and also synthesized for catalytic selectivity.
Uses:
- Zeolites are used as catalysts in petrochemical industries for cracking.
- Zeolite ZSM – 5 is used to convert alcohol directly into gasoline (petrol) by dehydrating them to give a mixture of hydrocarbons.
![]()
Question 10.
Give brief account of mechanism of enzyme catalysis with suitable diagrams.
Answer:
Mechanism of enzyme catalysis :
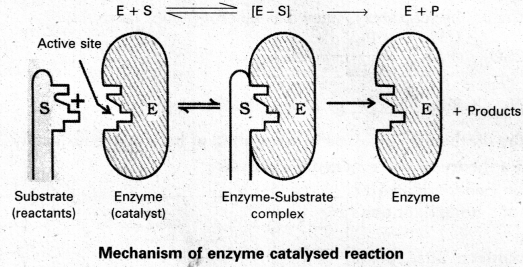
The enzyme – catalysed reactions may be considered to proceed in two steps.
Step 1 : Binding of substrate to enzyme to form an activated complex (ES≠).
E + S → E S≠
Step 2 : Decomposition of the activated complex to form product.
E S≠ → E + P
Question 11.
Discuss the factors that influence the catalytic activity of enzymes.
Answer:
Factors influencing the catalytic activity of enzymes :
- Highly specific nature: One catalyst catalyses only one reaction. Each enzyme is specific ‘ for a given reaction.
Eg : The enzyme urease catalyses the hydrolysis of urea only and not any other amide. - Highly active under optimum temperature: The rate of an enzyme reaction is maximum at a temperature called optimum temperature.
- The optimum temperature range for enzymatic activity is 298 – 310 K.
- Highly active under optimum pH : The rate of an enzyme – catalysed reaction is maximum at a particular pH called optimum pH which lies between 5 – 7.
- Increasing activity in presence of activators and co-enzymes : The enzymatic activity is increased in presence of co-enzymes (or) in presence of activators such as Na+, Mn+2 etc.,
- Influence of inhibitors and poisons: The inhibitors (or) poisons interact with the active functional groups on the enzyme surface and reduce or completely destroy the catalytic activity.
Question 12.
Name any six enzyme catalysed reactions. [A.P. Mar. 19]
Answer:
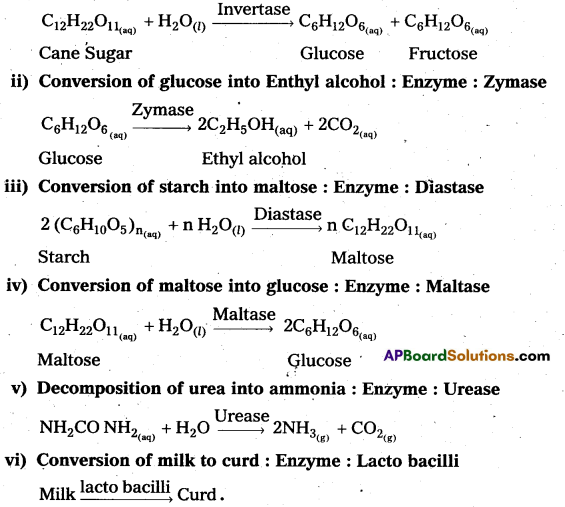
i) Inversion of Cane sugar : Enzyme : Invertase
Question 13.
What do you mean by activity and selectivity of catalysts ?
Answer:
Activity :
The ability of a catalyst in increasing the rate of reaction is defined as activity of catalyst.
- The activity of a catalyst depends upon the strength of chemisorption to a large extent.
- The reactants must get adsorbed reasonably strongly on to the catalyst to become reactive.
Eg : The catalystic activity increases from Group – 5 to Group – 11 for hydrogenation reactions.
The maximum activity being shown by 7 – 9 group metals.
Selectivity:
The selectivity of a catalyst is its-ability to direct a reaction to form specific products. The following reactions indicate the selectivity of heterogeneous catalysis.
Starting with H2 and CO, and using different catalysts, we get different products.
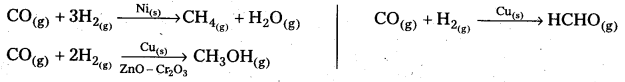
The action of a catalyst is highly selective in nature. A substance which acts as a catalyst in one reaction may fail to catalyse another reaction.
![]()
Question 14.
How are colloids classified on the basis of physical states of components ?
Answer:
On the basis of physical states of dispersed phase and dispersion medium colloidal solutions are classified into eight types.
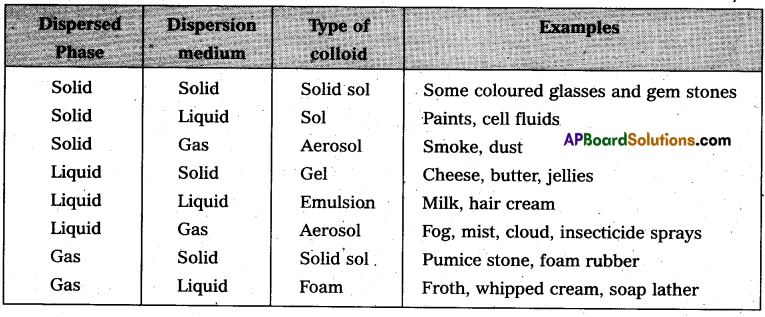
Question 15.
How are colloids classified on the basis of nature of the dispersion medium ?
Answer:
On the basis of nature of dispersion medium colloids are classified as follows.
- If the dispersion medium is ‘air’, the sols are called aerosols.
Eg : Smoke. - If the dispersion medium is ‘water’ the sols are called Hydrosol (or) aquasol.
Eg : starch solution. - If the dispersion medium is ‘alcohol’ then the sols are called alcosols.
Question 16.
How are colloids classified on the basis of interaction between dispersed phase and dispersion medium ?
Answer:
Lyophilic colloid : The colloidal solution in which the dispersed phase has great affinity to the dispersion medium is called a Lyophilic colloid or Lyophilic solution.
Ex: Starch solution.
The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid : The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution.
Ex: Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution.
Gold particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
![]()
Question 17.
What is the difference between a colloidal sol, gel, emulsion and a foam?
Answer:
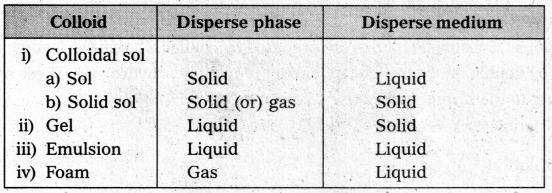
Question 18.
What are lyophilic and lyophobic sols? Compare the two terms in terms of stability and reversibility.
Answer:
Lyophilic colloid : The colloidal solution in which the dispersed phase has great affinity to the dispersion medium is called a Lyophilic colloid or Lyophilic solution.
Ex : Starch solution.
The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid : The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution.
Ex: Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution.
Gold particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
Question 19.
Name a substance whose molecules consist of lyophilic as well as lyophobic parts. Give its use in our daily life.
Answer:
- Associated colloids (or) micelles contains both lyophobic and lyophillic parts.
- Examples are soaps and synthetic detergents.
- The cleaning action of soap is due to the fact that soap molecule form a micelle around the oil droplet.
Question 20.
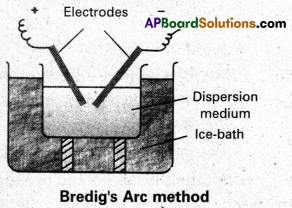
Describe Bredig’s arc method of preparation of colloids with a neat diagram.
Answer:
Electrical disintegration or Bredig’s Arc method : This process involves dispersion as well as condensation. Colloidal sols of metals such as gold, silver, platinum, etc., are prepared by this method. In this method, electric arc is struck between electrodes of the metal immersed in the dispersion medium. The intense heat produced vapourises the metal, which then condenses to form particles of colloidal size.
Question 21.
Name any four examples of preparation of colloids by chemical methods with necessary chemical equations.
Answer:
Chemical methods : Colloidal solutions are prepared by chemical reactions leading to the formation of species by double decompostion, oxidation, reduction or hydrolysis. These species then aggregate leading to the formation of sols.
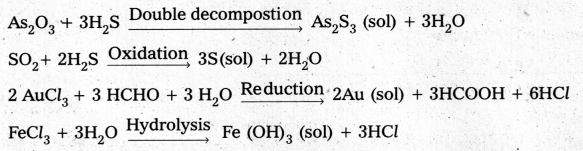
![]()
Question 22.
Describe the purification of colloidal solutions by the phenomenon of dialysis with a neat diagram. [A.P. Mar. 18]
Answer:
Colloidal solutions generally contain excess amount of electrolytes and some other soluble impurities. It is necessary to reduce the concentration of soluble impurities to a requisite minimum. The impurities presence required in traces.
“The process used for reducing the amount of impurities to a requisite minimum is known as purification of colloidal solution”.
Purification of colloidal solution by Dialysis :
Dialysis : The process of removing a dissolved substance from a colloidal solution using a suitable membrane is called dialysis.
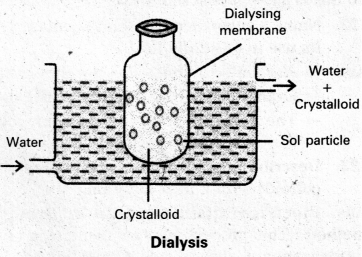
- In a true solution particles can pass through animal membrane (or) cellophane sheet (or) parchment paper but not colloidal particles.
- The apparatus used for the dialysis is called dialyser.
- A bag of suitable membrane containing the colloidal solution is suspended in a vessel containing a continuously flowing water.
- The molecules and ions diffuse through the membrane into the water and pure colloidal solution is left behind in the bag.
Question 23.
Explain the formation of micelles with a neat sketch.
Answer:
Mechanism of micelle formation: Let us take the example of soap solution. Soap is sodium or potassium salt of a higher fatty acid and may be represented as RCOC– Na+ (e.g., sodium strearate CH3 (CH2)16 COO– Na+, which is a major component of many bar soaps). When dissolved in water, it dissociates into RCOO– and Na+ ions. The RCOO– ions, however, consist of two parts – a long hydrocarbon chain R (also called non-polar ‘tail’) which is hydrophobic (water repelling), and a polar group COO–(also called polar-ionic ‘head’), which is hydrophilic (water loving).
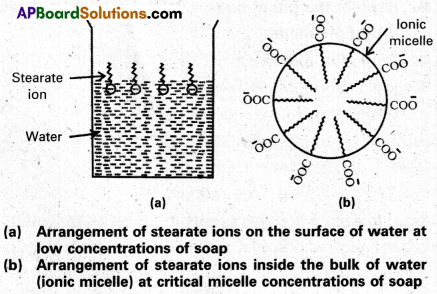
The RCOO– ions are, therefore, present on the surface with their COO– groups in water and the hydrocarbon chains (R) staying away from it and remain at the surface. But at critical micelle concentration, the COO– irons are pulled into the bulk of the solution and are aggregated to form a spherical shape with their hydrocarbon chains pointing towards the centre of the . sphere with COO– part remaining outward on the surface of the sphere. The aggregate thus formed is known as ‘ionic micelle’. These micelles may contain as many as 100 simple molecules.
Similarly, in case of detergents, e.g., sodium laurylsulphate. CH3 (CH2)11 SO3–Na+, the polar group is -SO3– along with the long hydrocarbon chain. Hence, the mechanism of micelle formation here also is same as that of soaps.
Question 24.
Action of soap is due to emulsification and micelle formation. Comment.
Answer:
Soap is sodium stearate, C17H35COONa, in water soap gives the ions stearate anion and sodium ion.
Cleaning action of soap : Soap anions form a micelle. The grease or dirt of the cloth are absorbed into the interior of the micelle. The tails of the anion are pegged into micelle and these micelle are washed away with the soap solution.
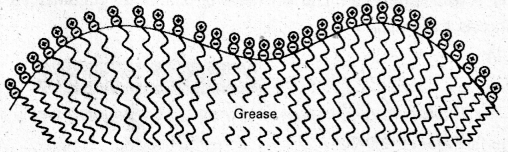
Soap thus, functions as an emulsifying agent for the water dirt emulsion. The emulsified grease or dirt is then washed away with soap solution.
![]()
Question 25.
Explain the phenomenon of Brownian movement giving reasons for the occurrence of this phenomena.
Answer:
Brownian movement: This is a kinetic property of colloidal solution.
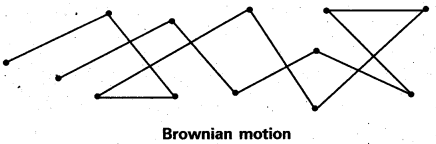
When a colloidal solution is examined by ultramicroscope, the colloidal particles are seem to be moving in a rapid zig – zag motion.
This rapid motion of colloidal particles is called Brownian movement. This motion is due to unequal bombardment of colloidal particles by molecules of dispersion medium. Smaller the colloidal particles the more rapid is the Brownian movement.
Question 26.
Name the four positively charged sols. .
Answer:
The following are the positively charged sols.
- Hydroated metallic oxide sols. Eg : Al2O3.xH2O, CrO3.xH2O etc.
- Basic dye stuffs Eg : Methylene blue sol.
- Hemoglobin (blood).
- Oxides Eg : TiO2 sol.
Question 27.
Name the four negatively charged sols.
Answer:
The following are the negatively charged sols.
- Metal sols Eg : Ag, Au-sols.
- Metallic suphides sols Eg : ArS3, CdS sols.
- Acid dye stuffs Eg: eosinol.
- Sols of starch, gum, clay etc.
Question 28.
Explain the terms helmholtz electrical double layer and zeta potential. What are their significancies in the colloidal solutions ?
Answer:
Helmholtz electrical double layer :
The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer.
- In a colloidal sol the charges of opposite signs on the fixed and diffused parts of the double layer results in a difference in potential between these layers. The potential difference between the fixed layer and the diffused layer of opposite charge is called electro kinetic potential (or) zeta potential.
- The above concepts applicable in colloidal solution in which the solid particles carry one kind of charge while liquid medium carries opposite charge to that of solids.
![]()
Question 29.
Explain with a neat sketch the phenomenon of electrophoresis.
Answer:
Electrophoresis : The existence of charge on colloidal particles is confirmed by electro-phoresis experiment.
When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal articles move towards one or the other electrode. The movement of colloidal particles under an applied emf is called “electrophoresis”.
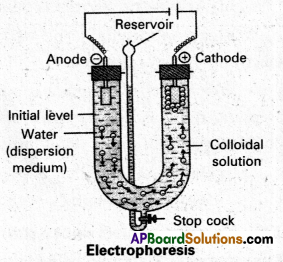
Positive charged particles move towards the cathode while negative charged particles towards anode.
Question 30.
Explain the following terms, i) Electrophoresis ii) Coagulation iii) Tyndall effect.
Answer:
Electrophoresis: When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode. The movement of colloidal particles under an applied emf is called “electrophoresis”.
Coagulation : The stability of the lyophilic sols is due to the presence of charge on the colloidal particles. If this charge is neutralised the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity. This process of settling down of colloidal particles is called coagulation (or) flocculation (or) precipitation.
Coagulation of lyophobic sols can be carried out by
i) Electrophoresis, ii) Boiling, iii) Adding Electrolytes, iv) Prolonged dialysis etc.
Tyndall effect : When light enters a colloidal solution, it is scattered by the large sized colloidal dispersed phase particles. Therefore when light passes through a solution we will be able to see the path of the light as a luminous beam. This is called Tyndall effect.
Question 31.
Explain the phenomenon observed
i) When a beam of light is passed through a colloidal sol.
Answer:
Tyndall effect : When light enters a colloidal solution, it is scattered by the large sized colloidal dispersed phase particles. Therefore when light passes through a solution we will be able to see the path of the light as a luminous beam. This is called Tyndall effect.
This is an optical property exhibited by colloidal solution. This phenomenon is clearly seen with a microscope placed at right angles to the path of light. Colloidal particles become self luminous due to absorption of light. A true solution does not show Tyndall effect.
![]()
ii) An electrolyte, NaCl is added to hydrated ferric oxide.
Answer:
When NaCl solution is added to the colloidal solution of hydrated ferric oxide coagulation takes place (precipitation formed).
The reason is that colloidal particles interact with ions (Na+, Cl–) carrying charge opposite to that present on themselves. This causes neutralisation of charges leading to their coagulation.
iii) An electric current is passed through a colloidal solution.
Answer:
Electrophoresis : the existence of charge on colloidal particles is confirmed by electrophoresis experiment.
When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode. The movement of colloidal particles under an applied emf is called “electrophoresis”.
Positive charged particles move towards the cathode while negative charged particles towards anode.
Question 32.
Describe cottrell smoke precipitator with a neat diagram.
Answer:
Aerial pollution by colloidal particles of smoke be prevented by electrical precipitation of smoke. Smoke is a colloidal solution of solid particles such as carbon, arsenic compounds, dust, etc., in air. The smoke, before it comes out from the chimney, is led through a precipitator containing plates having a charge opposite to that carried by smoke particles. The particles on coming in contact with these plates lose their charge and get precipitated. The particles thus settle down on the. floor of the chamber. The precipitator is called conttrels precipitator.
Question 33.
Among NaCl, Na2SO4, Na3PO4 electrolytes, which is more effective for coagulation of hydrated ferric oxide sol and why ?
Answer:
Among NaCl, Na2SO4, Na3PO4 electrolytes, Na3PO4 is more effective for coagulation of hydrated ferric oxide sol. This is explained by Hardy-Schulze rule.
Greater the valence of the coagulating ion added, the greater is its power to cause coagulation. This is known as Hardy-Schulze rule.
Coagulating ability order of the anions in the above salts is
PO4-3 > SO4-2 > Cl–.
Question 34.
Discuss how a lyophilic colloids protect a lyophobic colloids.
Answer:
Lyophilic colloids are much stable than lyophobic colloids. A very small amount of electrolyte can precipitate the lyophobic colloids. But their precipitation can be prevented by the addition of small amounts of lyophilic colloids. This lyophilic colloids form a protecting layer around the lyophobic colloids and protect them from precipitation (coagulation). Lyophilic colloids used for. this purpose are called protective colloids.
Ex : Gelatin, gum, starch sols are generally used as protective colloids.
![]()
Question 35.
Discuss the use of colloids in
i) Purification of drinking water ii) Tanning iii) Medicines.
Answer:
i) Purification of drinking water: The water obtained from natural sources often contains suspended impurities. Alum is added to such water to coagulate the suspended impurities and make water fit for drinking purposes.
ii) Tanning: Animal skins are colloidal in nature. When a skin, which has positively charged particles, is soaked in tannin, which contains negatively charged colloidal particles, mutual coagulation takes place. This results in the hardening of skin (leather). This process is termed as tanning. Chromium salts are also used in place of tannin.
iii) Medicines : Most of the medicines are colloidal in nature. For example argyrol is a silver sol used as an eye lotion. Colloidal antimony is used in curing kalaazar. Colloidal gold is used as intramuscular injection. Milk of magnesia, an emulsion, is used for stomach disorders. Colloidal medicines are more effective because they have large surface area and are therefore easily assimilated.
Question 36.
Define Gold Number.
Answer:
The capacity of the lyophilic colloid in protecting a lyophobic colloid is measured in terms of Gold Number, introduced by “Zigmondy”.
Gold Number:” It is the number of milligrams of lyophilic colloid that is to be added to 10 ml of standard gold sol to prevent its precipitation by the addition of 1ml of a 10% sodium chloride solution”.
Lesser in the gold number the greater is the protecting capacity. Gold number of a few colloids are given below.
Starch = 25 .
Gelatin = 0.005 – 0.01
Albumin =0.1 – 0.2
Question 37.
How do emulsifiers stabilize emulsion ? Name two emulsifiers.
Answer:
Emulsifying agent: The third substance which is added in small amounts to an emulsion to stabilize the emulsion is called emulsifying agent.
—> The emulsifying agent forms an interfacial film between suspended particles and the medium.
Eg : 1) Carbon black stabilizes water in oil type emulsions.
2) Casein and silica stabilize oil in water type emulsions.
Long Answer Questions
Question 1.
Explain the terms absorption, adsorption and sorption. Describe the different types of adsorption.
Answer:
Absorption : The uniform distribution of a substance through out the bulk of the solid substance is known as absorption.
Eg : Chalk stick dipped in ink.
Adsorption : The accumulation (or) concentration of a substance on the surface, rather than in the bulk of solid (or) liquid is known as adsorption.
Eg : Adsorption of gases like O2, H2, Cl2, etc., on charcoal
Sorption : In case of some substances both adsorption and absorption takes place. This phenomenon is called sorption.
Types of adsorption : On the basis of forces present between adsorbate and adsorbent molecules, adsorption classified into two types :
1) Physisorption, 2) Chemisorption.
1) Physisorption: If the forces that are responsible for the adsorption of adsorbate molecules on the surface of the adsorbent are weak vander waal’s for the adsorption is known as physisorption.
Eg : Adsorption of H2, on O2, on charcoal.
Characteristics :
- It is a weak adsorption.
- Enthalpy of adsorption is low (20 – 40 KJ / mole).
- It is reversible and occurs rapidly.
- It decrease with increase in temperature.
- It increase with increase in pressure.
- It is multilayered and not specific.
2) Chemisorption : If the forces that are responsible for the formation of adsorbate molecules on the source of adsorbent are chemical forces, the adsorption is called chemisorption.
Eg : Adsorption of H2 on Ni – metal surface.
Characteristics :
- It is a strong adsorption. ,
- Enthalpy of adsorption of high (80 – 240 KJ / mole).
- It is irreversible and occurs slowly.
- It increases with increase of temperature and finally decrease.
- Pressure has no effect on chemisorption.
- It is unilayered and specific.
![]()
Question 2.
Discuss the characteristics of physical adsorption.
Answer:
Characteristics of physical adsorption :
- Lack of specificity : Physisorption is not specific. In this adsorption the given surface of adsorbent doesnot show any preference for a particular gas as the forces present are variderwaal’s forces (Universal).
- Nature of adsorbate : The amount of gas adsorbed by a solid depends on the nature of gas. Easily liquefiable gases are readily adsorbed.
- The gas with high critical temperature value is easily liquefied and gets readily adsorbed.
- Reversible Nature : Physical adsorption is reversible and occurs rapidly at low temperatures.
- Surface area of adsorbent: The extent of adsorption increase with increase in surface area of adsorbent. .
- Finely divided metals and porous substances having large surface area are good adsorbents.
- Enthalpy of adsorption : Physisorption is an exothermic process but the enthalpy of adsorption is low (20 – 40 KJ/mole). This is due to the presence of Vander waal’s forces.
Question 3.
Discuss the characteristics of chemisorption. .
Answer:
- High specificity: Chemisorption is highly specific and it will only occur if there is some possibility of chemical bonding between adsorbate and adsorbent.
- Irreversibility : Chemisorption involves compound formation and is irreversible in nature.
- Chemisorption is exothermic and increases with increase of temperature.
- Surface area : Chemisorption increase with increase of surface area of the adsorbent.
- Enthalpy of adsorption : Enthalpy of chemisorption is high (80 – 240 KJ/mole).
Question 4.
Compare and contrast the phenomenon of physisorption and chemisorption.
Answer:
Characterstics of Physical adsorption :
Physisorption: If the forces that are responsible for the adsorption of adsorbate molecules on the surface of the adsorbent are weak vander waal’s for the adsorption is known as physisorption.
Eg : Adsorption of H2 on O2 on charcoal.
- Lack of specificity : Physisorption is not specific. In this adsorption the given surface of adsorbent doesnot show any preference for a particular gas as the forces present are vander waal’s forces (Universal). ‘
- Nature of adsorbate : The amount of gas adsorbed by a solid depends on the nature of gas.
- Easily liquefiable gases are readily adsorbed:
The gas with high critical temperature value is easily liquefied apd gets readily adsorbed. - Reversible Nature : Physical adsorption is reversible and occurs rapidly at low temperatures.
- Surface area of adsorbent: The extent of adsorption increase with increase in surface area of adsorbent.
- Finely divided metals and porous substances having large surface area are good adsorbents. .
- Enthalpy of adsorption : Physisorption is an exothermic process but the enthalpy of adsorption is low (20 – 40 KJ/mole). This is due to the presence of Vander waal’s forces.
2) Chemisorption : If the forces that are responsible for the formation of adsorbate molecules on the source of adsorbent are chemical forces, the adsorption is called chemisorption.
Eg : Adsorption of H2 on Ni – metal surface.
- High specificity: Chemisorption is highly specific and it will only occur if there is some possibility of chemical bonding between adsorbate and adsorbent.
- Irreversibility : Chemisorption involves compound formation and is irreversible in nature.
- Chemisorption is exothermic and increases with increase of temperature.. ,
- Surface area : Chemisorption increase with increase of surface area of the adsorbent.
- Enthalpy of adsorption : Enthalpy of chemisorption is high (80 – 240 KJ/mole).
![]()
Question 5.
What is an adsorption isotherm ? Discuss the phenomenon of adsorption of gases on solids with the help of Freundlich adsorption isotherm.
Answer:
Adsorption Isotherm : The variation in the amount of gas adsorbed by the adsorbent with pressure at constant temperature can be expressed by means of a curve known as adsorption Isotherm.
- Freundlich adsorption isotherm equation is
\(\frac{\mathrm{x}}{\mathrm{m}}\) = k, P1/n
x = mass of the gas adsorbed
m = mass of the adsorbent
P, k and n are constants. - k and n depend on the nature of the adsorbent and the gas at a particular temperature.
- The relation ship is generally represented in the form of a curve where x/m is plotted against pressure.
- These curves indicate that at a fixed pressure there is a decrease in physical adsorption with rise of temperature.
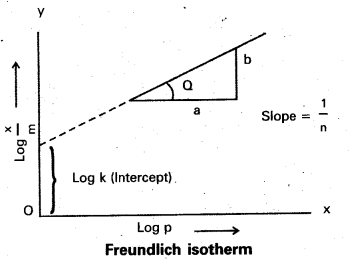
\(\frac{\mathrm{x}}{\mathrm{m}}\) = k.P1/4
Applying logarithm
Log \(\frac{\mathrm{x}}{\mathrm{m}}\) = log k + \(\frac{\mathrm{1}}{\mathrm{n}}\) log p. - The validity of Freundlich isotherm can be verified by plotting log \(\frac{\mathrm{x}}{\mathrm{m}}\) on y-axis and log P on x-axis. If it is a straight line then the isotherm is valid.
- The slope of the straight line give the value of \(\frac{\mathrm{1}}{\mathrm{n}}\)
- The intercept on the y-axis gives the value of log k.
Question 6.
Give a detailed account of applications of adsorption.
Answer:
Applications of adsorption : The phenomenon of adsorption funds a number of applications. Important ones are listed here.
- Production of high vacuum: The traces of air still remaining in the vessel evacuated by a vacuum pump to give high vacuum can be adsorbed by charcoal.
- Gas masks : Gas mask, a device which consists of activated charcoal or mixture of adsorbents is usually used by coal miners to adsorb poisonous gases during breathing.
- Control of humidity : Silica gel and alumina gel are used as adsorbents for removing mixture and controlling humidity of air in rooms.
- Removal of colouring matter from solutions : Animal charcoal removes colours of impure coloured solutions by adsorbing impurities responsible for the colour.
- Separation of inert gases : Due to the difference in degree of adsorption of gases by charcoal, a mixture of noble gases can be separated by adsorption on coconut charcoal at different temperatures.
- In curing diseases : A number of drugs kill germs by getting themselves adsorbed on germs.
- Froth floatation process : A low grade sulphide ore is concentrated by separating silica and other earthy matter by this method using pine oil and frothing agent.
- Adsorption indicators : Surfaces of certain precipitates such as silver halides have the property of adsorbing some dyes like eosin, fluorescein, etc. and thereby producing a characteristic colour change at the end point in argentometric titrations.
- Chromatographic analysis : Chromatographic analysis based on the phenomenon of adsorption finds a number of applications in analytical and industrial methods.
![]()
Question 7.
What is catalysis ? How is catalysis classified ?* Give four examples for each type of catalysis.
Answer:
Catalysis: A substance which alters the rate of a chemical reaction without itself being consumed in the process, is called a catalyst.
The action of catalyst in altering the rate of a chemical reaction is called catalysis.
Types of catalysis : Catalysis is classified into two types as .
a) Homogeneous catalysis and
b) Heterogeneous catalysis.
Homogeneous catalysis : The catalytic process in which the catalyst is present in the same phase as that of reactants, is known as homogeneous catalysis.
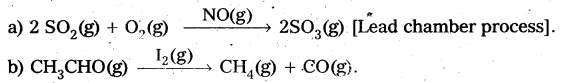
Heterogeneous catalysis: The catalytic process in which the catalyst is present in a phase different from that of reactarts is known as heterogeneous catalysis.
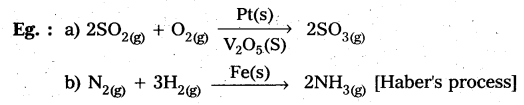
Question 8.
Discuss the mechanism of heterogeneous catalysis.
Answer:
Adsorption theory of heterogeneous catalysis :
Adsorption theory explains the mechanism of heterogeneous catalysis.
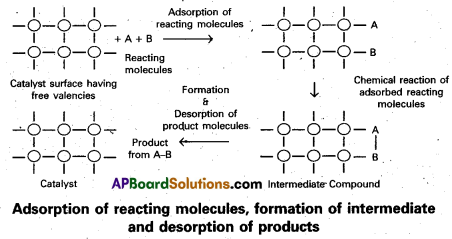
- The modern adsorption theory is the combination of the intermediate compound formation theory and the old adsorption theory.
- The mechanism of catalysis involves five steps.
- Diffusion of the reactants to the surface of the catalyst.
- Adsorption of the reactant molecules on the surface of the catalyst.
- Occurrence of chemical reaction on the catalyst’s surface through formation of an intermediate.
- Desorption of reaction products from the catalyst surface, and thereby making the surface available again for more reaction to occur.
- Diffusion of reaction products away from the catalyst’s surface.
Question 9.
What are enzymes ? Explain in detail the enzyme catalysis, with necessary examples.
Answer:
Enzymes are complex nitrogenous organic compounds which are produced by living plants and animals.
- These act as specific catalysts in biological reactions.
- These catalyse the numerous reactions that occur in the bodies of animals and plants to maintain the life process.
Mechanism of enzyme catalysis :
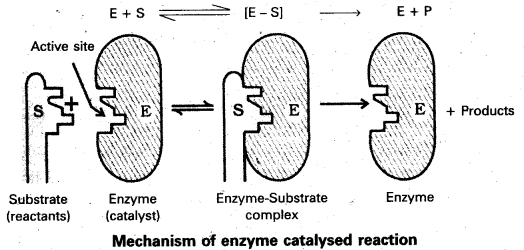
The enzyme – catalysed reactions may be considered to proceed in two steps.
Step 1 : Binding of substrate to enzyme to form an activated complex (ES≠)
E + S → ES≠
Step 2 : Decomposition of the activated complex to form product.
ES≠ → E + P
Factors influencing the catalytic activity of enzymes :
- Highly specific nature: One catalyst catalyses only one reaction. Each enzyme is specific for a given reaction.
Eg : The enzyme urease catalyses the hydrolysis of urea only and not any other amide - Highly active under optinum temperature: The rate of an enzyme reaction is maximum at a temperature called optimum temperature.
- The optimum temperature range for enzymatic activity is 298 – 310 K.
- Highly active under optimum pH : The rate of an enzyme – catalysed reaction is maximum at a particular pH called optimum pH which lies between 5-7
- Increasing activity in presence of activators and co-enzymes : The enzymatic activity is increased in presence of co-enzymes (or) in presence of activators such as Na+, Mn+2 etc.,
- Influence of inhibitors and poisons: The inhibitors (or) poisons interact with the active functional groups on the enzyme surface and reduce or completely destroy the catalytic activity.
i) Inversion of Cane sugar: Enzyme : Invertase
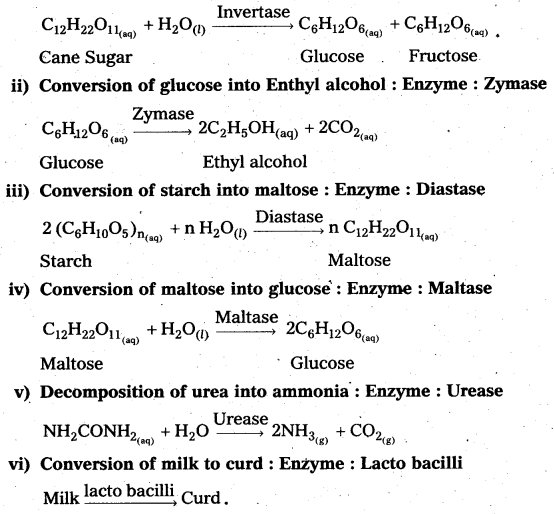
![]()
Question 10 & 11.
What are colloidal solutions ? How are they classified ? Give examples.
Answer:
A heterogeneous system in which one substance is dispered as large particles in another substance is called colloidal solution.
- In colloidal solution the particle size of the dispersed phase is of the order of 1 mμ – 1 μ where as in true solution the particle size of the solute are the order of mp or less.
- Colloidal solution is a heterogeneous binary system where as true solution is a homogeneous binary system.
On the basis of physical states of dispersed phase and dispersion medium colloidal solutions are classified into eight types.
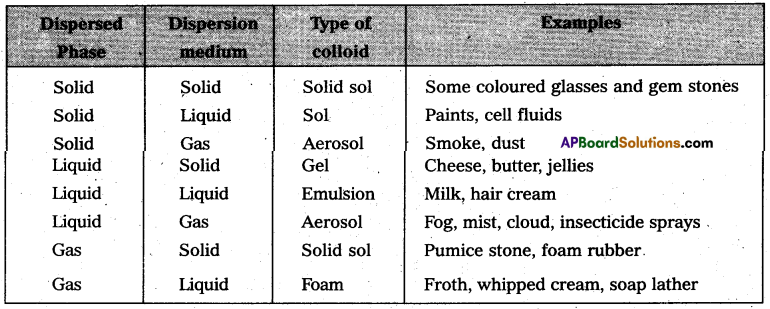
On the basis of nature of dispersion medium colloids are classified as follows.
If the dispersion medium is ‘air’, the sols are called aerosols.
Eg : Smoke.
If the dispersion medium is ‘water’ the sols are called Hydrosol (or) aquasol.
Eg: starch solution.
If the dispersion medium is ‘alcohol’ then the sols are called alcosols.
The colloidal solution in which the dispersed phase has great affinity to the dispersion medium is called a Lyophilic colloid or Lyophilic solution.
Ex : Starch solution.
The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid : The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution. Ex : Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution.
Gold particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
Question 12.
How are colloids classified on the basis of the nature of interaction between a dispersed phase and a dispersion medium ? Describe an important characteristic of each class. Which of the sols need stabilising agents for preservation ?
Answer:
The colloidal solution in which the dispersed phase has great affinity to the dispersion medium is called a Lyophilic colloid or Lyophilic solution.
Ex : Starch solution.
The starch paste when dissolved in hot water, with stirring, the starch solution is formed. The starch particles (dispersed phase) has great affinity to water molecules (dispersion medium). So starch solution is a lyophilic solution or lyophilic colloid.
Lyophobic colloid : The colloidal solution in which there exists not much affinity between the dispersed phase and dispersion medium, it is called a Lyophobic colloid or Lyophobic solution.
Ex: Gold solution.
Gold rods are placed in water containing alkali. Electric arc is applied between gold rods. The gold particles dissolves in water, to give gold solution.
Gold particles (dispersed phase) have not much affinity towards water (dispersion medium). So this is a Lyophobic solution or Lyophobic colloid.
Lyophilic colloids are much stable than lyophobic colloids. Avery small amount of electrolyte can precipitate the lyophobic colloids. But their precipitation can be prevented by the addition of small amounts of lyophilic colloids. This lyophilic colloids form a protecting layer around the lyophobic colloids and protect them from precipitation (coagulation). Lyophilic colloids used for this purpose are called protective colloids.
Ex : Gelatin, gum, starch sols are generally used as protective colloids.
![]()
Question 13.
What are micelles ? Discuss the mechanism of micelle formation and cleaning action of soap.
Answer:
Micelles : Some substances which at low concentrations behave as normal strong electrolytes, but at high concentrations exhibit colloidal behaviour due to formation of aggregates. The aggregated particles thus formed are called micelles.
Eg : Stearate ions (C17H35COO–) associate together in high concentration, in a solution of soap in water and forms a micelle.
Mechanism of micelle formation : Let us take the example of soap solution. Soap is sodium or potassium salt of a higher fatty acid and may be represented as RCOC– Na+ (E.g., sodium strearate CH3 (CH2)16 COO– Na+, which is a major component of many bar soaps). When dissolved in water, it dissociates into RCOO– and Na+ ions. The RCOO– ions, however, consist of two parts – a long hydrocarbon chain R (also called non-polar ’tail) which is hydrophobic (water repelling), and a polar group COO–(also called polar-ionic ‘head), which is hydrophilic (water loving).
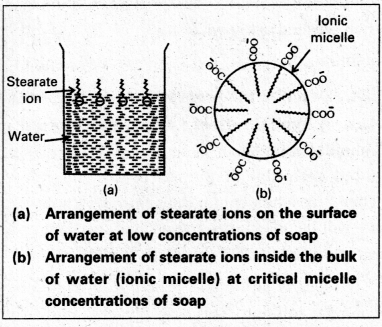
The RCOO– ions are, therefore, present on the surface with their COO– groups in water and the hydrocarbon chains (R) staying away from it and remain at the surface. But at critical micelle concentration, the COO– irons are pulled into the bulk of the solution and are aggregated to form a spherical shape with their hydro¬carbon chains pointing towards the centre of the sphere with COO- part remaining outward on the surface of the sphere. The aggregate thus formed is known as ‘ionic micelle’. These micelles may contain as many as 100 simple molecules.
Similarly, in case of detergents, E.g., sodium laurylsulphate. CH3 (CH3)11 SO3–Na+, the polar group is -SO3–along with the long hydrocarbon chain. Hence, the mechanism of micelle formation here also is same as that of soaps.
Soap is sodium stearate, C17H35COONa, in water soap gives the ions stearate anion and sodium ion.
C17H35COONa → C17H35COO– + Na+ (Stearate ion)
The anion of soap = C17H35COO–
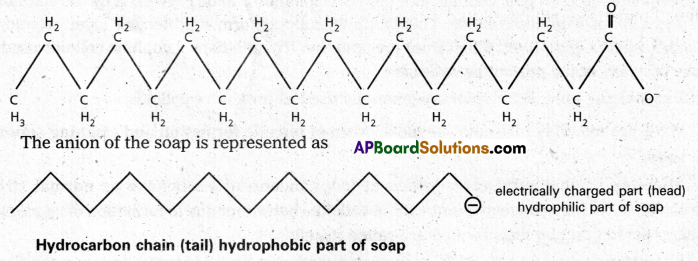
Cleaning action of soap : Soap anions form a micelle. The grease or dirt of the cloth are absorbed into the interior of the micelle. The tails of the anion are pegged into micelle and these micelle are washed away with the soap solution.
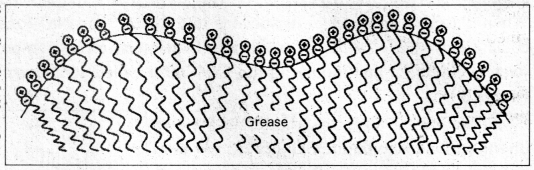
Soap thus, functions as an emulsifying agent for the water dirt emulsion. The emulsified grease or dirt is then washed away with soap solution.
Question 14.
Describe the properties of colloids with necessary diagrams wherever necessary.
Answ:
i) Tyndall effect : When light enters a colloidal solution, it is scattered by the large sized colloidal dispersed phase particles.. Therefore when light passes through a solution we will be able to see the path of the light as a luminous beam. This is called Tyndall effect.
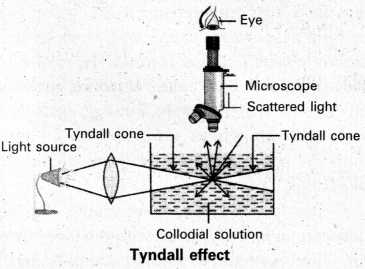
This is an optical property exhibited by colloidal solution. This phenomenon is clearly seen with a microscope placed at right angles to the path of light. Colloidal particles become self luminous due to absorption of light.
A true solution does not show Tyndall effect.
ii) Brownion movement: This is a kinetic property of colloidal solution.
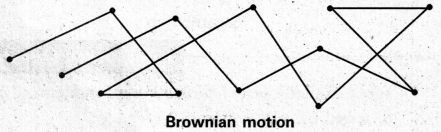
When a colloidal solution is examined by ultramicroscope, the colloidal particles are seem to be moving in a rapid zig-zag motion.
This rapid motion of colloidal particles is called Brownian movement.
This motion is due to unequal bombardment of colloidal particles by molecules of dispersion medium. Smaller the colloidal particles the more rapid is the Brownian movement.
iii) Charge on colloidal particles : The charge on the colloidal particles is due to electron capture by sol particles during electro dispersion of metals and due to preferrential adsorption ions from solution (of) due to formulation of electrical double layer.
When a dilute AgNO3 solution is added to a dilute KI solution taken in excess the precipitated AgI adsorbs I– ions present in excess and a negatively charged AgI colloidal solution is formed. However when dilute KI solution is added to dilute AgNO3 solution taken in excess, AgI precipitate adsorbs Ag+ ions present in excess and a positively charged Agl colloidal solution is formed. Generally the ion common to dispersed particle is adsorbed.
AgI/I–
Negatively charged
AgI/Ag+
Positively charged.
iv) Zeta Potential: In a colloidal sol the charges of opposite signs on the fixed’and diffused parts of the double layer results in a difference in potential between these layers. The potential difference between the fixed layer and the diffused layer of opposite charge is called electro kinetic potential (or) zeta potential.
v) electrophoresis : When electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode. The movement of colloidal particles under an applied emf is called “electrophoresis”.
If the movement of colloidal particles is arrested by some suitable means, the dispersion medium moves in opposite direction. This phenomenon is termed “electro osmosis”.
vi) Coagulation : The stability of the lyophilic sols is due to the presence of charge on the colloidal particles. If tins charge is neutralised the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity. This process of settling down of colloidal particles is called coagulation (or) flocculation (or) precipitation.
![]()
Question 15.
What are emulsions ? How are they classified ? Describe the applications of emulsion. [T.S. Mar. 18, 16]
Answer:
Emulsion: The colloidal system in which a dispersion of finely divided droplets of a liquid in another liquid medium is called Emulsion.
Ex: Milk. .
In Milk, the droplets of liquid fat are dispersed in water. This is an example for oil in water type emulsion.
Classification of emulsions : Emulsions are classified, into two classes. These are
a) oil in water (O/W) and ‘
b) water in oil (W/O), (O = Oil; W = Water).
These emulsions are classified as such depending on which is dispersed phase and which is dispersion medium.
a) Oil in Water (O/W) type emulsions : In this type of emulsions, the dispersed phase is oil and the dispersion medium is water.
Ex : Milk, liquid, fat (oil) in water.
Vanishing cream; fat in water.
b) Water in Oil (W/O) type emulsions : In this type of emulsions, the dispersed phase is water and the dispersion medium is oil.
Ex : Stiff greases : water in lubrication oils
Cod liver oil : water in cod liver oil
Cold cream : water in fat.
Applications of Emulsions : Emulsions are useful
- In the digestion of fats in intestines.
- In washing processes of clothes and crockery.
- In the preparation of lotions, creams, ointments in pharmaceutical and cosmetics.
- In the extraction of metals (froth flotation).
- In the conversion of cream into butter by churning.
- To break oil and water emulsions in oil wells.
- In the preparation of oily type of drugs for easy adsorption to the body.
Intext Questions
Question 1.
Write any two characteristics of Chemisorption. .
Solution:
- High specificity: Chemisorption; is highly specific and it will only occur when adsorbent and adsorbate molecules can chemically react with each other.
Eg.: oxygen is adsorbed on metals by oxide formation. - Surface area : Chemisorption increases with increase in surface area of the adsorbent.
Question 2.
Why does physisorption decrease with the increase of temperature ?
Solution:
Physisorption is an exothermic process :
Solid (Adsorbent) + Gas (Adsorbate) ⇌ Gas adsorbed on solid + Heat
When temperature is increased, the equilibrium shifts towards the backward direction to neutralise the effect of the change (Le-Chatelier’s principle). So, physisorption decreases with increase in temperature.
Question 3.
Why are finely powdered substances more effective adsorbents than their non powdered crystal forms ? .
Solution:
Finally divided substances provide increased surface area for adsorption which is not available to such extent in their crystalline forms. That’s why the powdered forms are more effective than crystalline forms for the purpose of adsorbents.
Question 4.
Hydrogen used in Haber’s process is obtained by reacting methane with steam in presence of NiO as catalyst. The process is known as steam reforming. Why is it necessary to remove CO formed in steam reforming when ammonia is obtained by Haber’s process ?
Solution:
Carbon monoxide (CO) acts as a poison for catalyst iron and promoter molybdenum in Haber’s process, i.e., the efficiency of catalyst and promoter is decreased. It also combines with Fe to form iron carbonyl, Fe(CO)5 which interfere in the production of ammonia. Hence, CO must be removed from the reaction mixture.
![]()
Question 5.
Why is ester hydrolysis slow in the beginning but is fast after sometime ?
Solution:
The chemical equation for ester hydrolysis is as follows :
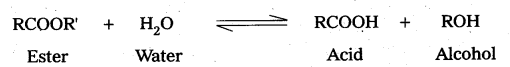
Carboxylic acid produced during hydrolysis releases H+ ions in the solution which act as catalyst (auto-catalysis) for the reaction. Therefore, ester hydrolysis is slow in the beginning but becomes faster after sometime.
Question 6.
What is role of desorption in the process of adsorption catalysis.
Solution:
The reaction products formed on the catalyst surface get detached from the surface as a result of desorption, thereby making the surface available again for more reactant particles.
Question 7.
What modification can you suggest in the Hardy-Schulze law ?
Solution:
According to Hardy Schulze law, the ions carrying charge opposite to the charge on sol particles neutralise their charge and thus cause their coagulation or precipitation. But actually, the sol carrying these ions also get coagulated since the ions neutralize their charge. So, Hardy Schulze law can be modified as :
“When equimolar proportions of two oppositely charged sols are mixed, they mutually neutralize their charge and both get coagulated”.
Question 8.
Why is it essential to wash the precipitate in gravimetric chemical analysis with wash liquid before drying and weighing it quantitatively ?
Solution:
Some of the reactant ions my be adsorbed or may adhere to the surface of the particles of the precipitate formed during an ionic reaction. In order to remove these reactant ions, the precipitate should.be washed with water. If this is not done, an error may be produced during quantitative analysis.