Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material Lesson 6(b) Group-16 Elements Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material Lesson 6(b) Group-16 Elements
Very Short Answer Questions
Question 1.
Why is dioxygen a gas but sulphur a solid?
Answer:
Dioxygen is a gas but sulphur a solid.
Explanation:
- Due to small atomic size and high electronegativity oxygen forms Pπ – Pπ multiple bond and exists as O2 molecules held together by weak vander waal’s forces. Thus oxygen exists as a gas at room temperature.
- Due to large atomic size and less electronegativity sulphur forms strong S—S single bonds and exists as S8 molecules with puckered ring structure. Hence sulphur is a solid at room temperature.
Question 2.
What happens when
a) KClO3 is heated with MnO2
b) O3 is passed through KI soultion
Answer:
a) When KClO3 is heated with Mn02 and liberates oxygen gas.
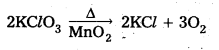
b) O3 is passed through KI solution I2 is liberated
2KI + O3 + H2O → 2KOH + I2 + 2O2
![]()
Question 3.
Give two examples each for amphoteric oxides and neutral oxides.
Answer:
- Examples of amphoterc oxides – Al2O3, SiO2, PbO.
- Examples of neutral oxides – CO, NO and N2O.
Question 4.
Oxygen generally exhibits an oxidation state of -2 only while the other members of the group show oxidation states of +2, +4 and +6 also – explain.
Answer:
- Oxygen exhibits -2 oxidation state due to its high electronegativity. The tendency of exhibiting -2 oxidation state decrease down the group.
- Due to decrease of electronegativity down the group the other elements exhibit +2, +4 and +6 oxidation states also.
Question 5.
Write any two compounds, in which oxygen shows an oxidation state different from -2. Give the oxidation states of oxygen in them.
Answer:
OF2 and O2 F2 are two compounds in which oxygen shows an oxidation state different from-2.
- In OF2 the oxidation state of oxygen is +2
- In O2F2 the oxidation state of oxygen is +1.
Question 6.
Oxygen molecule has the formula O2 while sulphur has S8 – explain.
Answer:
Due to small atomic size and high electronegativity oxygen forms Pπ – Pπ multiple bond and exists as O2 molecule.
Due to large atomic size and less electronegativity sulphur forms strong S – S single bonds and exists S8 molecule.
Question 7.
Why is H2O a liquid while H2S is a gas ?
Answer:
H2O is liquid due to the presence of intermolecular hydrogen bonding. While H2S is gas because it is not having such type of bonding.
![]()
Question 8.
H2O is neutral while H2S is acidic – explain.
Answer:
H2O is neutral while H2S is acidic.
Reason: The O-H bond dissociation Enthalpy is greater than the S – H bond dissociation Enthalpy.
Question 9.
Name the most abundant element present in earth’s crust.
Answer:
The most abundant element present in earth’s crust is oxygen (about 46.6%).
Question 10.
Which element of group-16 shows highest catenation ?
Answer:
Sulphus shows highest catenation among group – 16 elements and exists as S9 molecule with puckered ring structure.
Question 11.
Among the hydrides of chalcogens, which is most acidic and which is most stable ?
Answer:
- Among hydrides of chalcogens, H2Te is most acidic.
- Among hydrides of chalcogens, H2O is most stable.
Question 12.
Give the hybridization of sulphur in the following.
a) SO2
b) SO3
c) SF4
d)SF6
Answer:
a) Hybridisation of’S’ in SO2 is Sp2
b) Hybridisation of ‘S’ in SO3 is Sp2
c) Hybridisation of ‘S’ in SF4 is Sp3d
d) Hybridisation of ‘S’ in SF6 is Sp3d2
Question 13.
Write the names and formulae of any two oxyacids of sulphur. Indicate the oxidation state of sulphur in them.
Answer:
- Peroxy mono sulphuric acid – H2SO5 ‘S’ oxidation state +6 .
- Peroxy di sulphuric acid – H2S2O8 ‘S’ oxidation state +6
![]()
Question 14.
Explain the structures of SF4 and SF6.
Answer:
Structure of SF4:
- It SF4 ‘S’ undergoes sp3d hybndisation.
- It has trigonal bipyramidal structure in which one of the equitorial positions is occupied by a lone pair of electrons. This geometry is also known as see – saw geometry.
Structure of SF6:
- In SF6 ‘S’ undergoes sp3d2 hybridisation.
- It has octahedral structure.
Question 15.
Give one example each for
a) a neutral oxide
b) a peroxide
c) a super oxide
Answer:
a) CO, N2O are neutral oxides.
b) Na2O2, BaO2 are pernxides.
c) KO2, RbO2 are super oxides.
Question 16.
What is tailing of mercury? How is it removed? [A.P. & T.S. (Mar. 15)]
Answer:
Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
It is removed by shaking it with water which dissolves Hg2O.
Question 17.
Write the principle involved in the quantitative estimation of ozone gas.
Answer:
When ozone reacts with an excess of Kl solution buffered with a borate buffer (pH 9.2) iodine is liberated which can be titrated against a standard solution of sodium thiosulphate. In this way O3 is estimated quantitatively.
![]()
Question 18.
Write the structure of ozone.
Answer:
Structure of Ozone:

- O3 is angular molecule with bond angle 117°
- O – O bond length is 128 pm.
Question 19.
SO2 can be used as an anti-chior. Explain.
Answer:
SO2 gas is used as anti-chlor. Anti-chior means the substance which removes the excess of on clothes. SO2 reacts with chlorine in presence of charcoal to give suiphuryl chloride
SO2(g) + Cl2(g) → SO2Cl2l
Question 20.
How is ozone detected ?
Answer:
Ozone is a pale blue gas, dark blue liquid and violet black solid and it has characterstic smell.
- It is detected by the reaction with ‘Hg’ which is called as tailing of mercury.
2Hg + O3 → Hg2O + O2 - Ozone turns benzidene paper to brown colour.
Question 21.
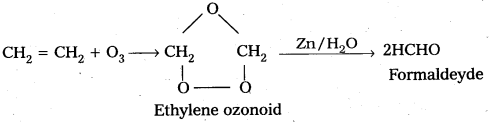
How does ozone react with Ethylene ?
Answer:
Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
![]()
Question 22.
Out of O2 and O3, which is paramagnetic ?
Answer:
- O2 is paramagnetic due to presence of unpaired electrons
- O3 (gaseous) is diamagnetic due to absence of unpaired electrons.
Question 23.
Between O3 and O2, ozone is a better oxidizing agent – why ?
Answer:
Ozone is next to fluorine in the oxidising capacity. It is best oxidising agent than O2. (Fluorine is the powerful oxidising agent). Ozone liberates nascent oxygen easily.
Question 24.
Write any two uses each for O3 and H2SO4.
Answer:
Uses of O3:
- Ozone is used in sterilisation of water.
- Ozone is used in manufacture of artificial silk and camphor etc.
- Ozone is used to identify unsaturation in carbon compounds.
Uses of H2SO4:
- H2SO4 is used in the manufacture of fertilisers.
- H2SO4 is used in petrol refining.
- H2SO4 is used in detergent industry.
Question 25.
Which form of sulphur shows paramagnetism ?
Answer:
In vapour state sulphur partly exists as S2 molecule which has two unpaired electrons in the antibonding π (π*)orbitals like O2. Hence exhibits paramagnetism.
Question 26.
How is the presence of SO2 detected ?
Answer:
SO2 has a pungent odour SO2 presence can be detected by the following tests.
- SO2 changes the colour of acidified potassium dichromate solution from orange to green.
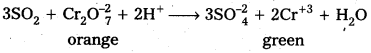
- SO2 decolourises acidified KMnO4 solution.
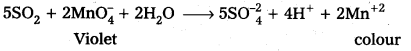
![]()
Question 27.
Why are group – 16 elements called chalcogens ?
Answer:
Chalcogens means mineral forming (or) ore forming elements. Most of elements exist in earth crust as oxides, sulphides, selinides, telurids etc. So Group – 16 elements are called as chalcogens.
Question 28.
Among chalcogens, which has highest eletronegativity and which has highest electron gain enthalpy?
Answer:
- Among chalcogens Oxygen has high electronegativity.
- Among chalcogens Sulphur has high electron gain Enthalpy.
Question 29.
Which hydride of group – 16 has highest boiling point and weakest acidic character ?
Answ:
- Among group – 16 hydrides water (H2O) has high bioling point.
- Among group – 16 hydrides water (H2O) has weakest acidic, character.
Short Answer Questions
Question 1.
Justify the placement of O, S, Se, Te and Po in the same group of the periodic table in terms of electronic configuration, oxidation states and hydride formation.
Answer:
1) Electronic configurations :
Oxygen (O) – [He] 2s2 2p2
Sulphur (S) – [Ne] 3s2 3p2
Selenium (Se) – [Ar] 3d10 4s24p4
Tellurium (Te) – [Kr] 4d10 5s2 sp4
Polonium (Po) – [Xe] 4f14 5d10 6s2 6p4
All the above elements has general outer electronic configuration ns2np4.
2) Oxidation states :
All the gives elements (chalcogens) exhibits common oxidation state of-2
O-2, S-2, Se-2 etc.
3) Hydride formation:
All these elements (chalcogens) forms hydrides of type EH2(E = chalcogen)
Eg : H2O, H2S, H2Se, H2Te, H2Po.
The above mentioned concepts evident that the elements O, S, Se, Te and Po are present in the same group of periodic table.
![]()
Question 2.
Describe the manufacture of H2SO4 by contact process.
Answer:
Manufacture of H2SO4 by contact process:
Manufacturing of H2SO4 involves three main steps.
Step – 1.
SO2 production : The required SO2 for this process is obtained by burning S(or) Iron pyrites in oxygen.
S + O2 → SO2
4FeS2 + 15O2 → 2Fe2O3 + 8SO3
Step – 2
SO3 formation: SO2 is oxidised in presence of catalyst with atmosphric air to form SO3
![]()
Step – 3
Formation of H2SO4: SO3 formed in the above step absorbed in 98% H2SO4 to get oleum (H2S2O7). This oleum is diluted to get desired concentration of H2SO4.
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → 2H2SO4
Question 3.
How is ozone prepared ? How does it react with the following? [Mar. 14]
a) PbS
b) KI
c) Hg
d) Ag
Answer:
Preparation of Ozone:
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3 ∆ H° = 142kJ/mole
- The formation of ozone is an endothermic reaction.
- It is necessary to use silent electric discharge in the preparation of O3 to prevent its decomposition
a) Reaction with PbS : Black lead suiphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2
b) Reaction with KI: Moist Kl is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 -→ 2KOH + I2 + O2
c) Reaction with Hg : Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2 O + O2
It is removed by shaking it with water which dissolves Hg2O.
d) Reaction with Ag : Ag metal oxidised to Ag2O (Ag metal is tarnished):
2Ag + O3 → Ag2O + O2
![]()
Question 4.
Write a short note on the allotropy of sulphur.
Answer:
The important allotropes of sulphur are
a) yellow rhombic (α. sulphur)
b) Monoclinic (β – sulphur)
- The stable from is α-sulphur (at room temperature)
Rhombic sulphur (α – Sulphur):
- Colour : Yellow. ,
- Melting point: 385.8K.
- Specific gravity : 2.06.
- It is insouble in water and partially soluble in alcohol, benzene etc. and readily soluble in CS2.
Monoclinic sulphur (β – Sulphur):
- Melting point: 392K
- Specific gravity : 1.98.
- It is soluble in CS2.
- Rhombic sulphur transforms to monoclinic sulphur by heating above 369K. This temperature is called transition temperature.
Question 5.
How does SO2 react with the following ?
a) Na2SO3(aq)
b) Cl2
c) Fe+3 ions
d) KMnO4
Answer:
a) Sodium sulphite (aq) reacts with So2 to form sodium hydrogen sulphite.
Na2SO3 + H2O + SO2 → 2NaHSO3
b) SO2 as reacts with chlorine gas in the presence of charcoal to form sulphuryl chloride.
SO2(g) + Cl2(g) → SO2Cl2(l)
c) Fe+3 ions are reduced to Fe+2 ions by SO2.
2Fe+3 + SO2 + 2H2O → 2Fe+2 + SO-24 + 4H+
d) SO2 gas decolourises acidified potassium permanganate (VII) solution.
5SO2 + 2MnO–4 + 2H2O → 5SO-24 + 4H++ 2Mn+2
Question 6.
Starting from elemental sulphur, how is H2SO4 prepared ?
Answer:
Manufacture of H2SO4 by contact process :
Manufacturing of H2SO4 involves three main steps.
Step – 1.
SO2 production : The required SO2 for this process is obtained by burning S(or) Iron pyrites in oxygen.
S + O2 → SO2
4FeS2 + 15O2 → 2Fe2O3 + 8SO3
Step – 2
SO3 formation: SO2 is oxidised in presence of catalyst with atmosphric air to form SO3
![]()
Step – 3
Formation of H2SO4: SO3 formed in the above step absorbed in 98% H2SO4 to get oleum (H2S2O7). This oleum is diluted to get desired concentration of H2SO4.
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → 2H2SO4
![]()
Question 7.
Describe the structures (shapes) of SO-24 and SO3.
Answer:
Structure of SO3:
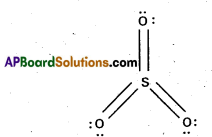
- In SO3 sulphur undergoes sp2 hybridisation.
- Shape : Trigonal planait s
- Bondangle: 120°.
- S -0 bond length: 143 pm.
Structure of SO-24:
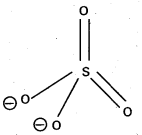
- In SO-24 sulphur undergo sp3 hybridisation.
- Shape : Tetrahedral.
- It has several resonance structures.
- In this two Pπ – dπ bonds are present.
Question 8.
Which oxide of sulphur can act as both oxidizing and reducing agent? Give one example each.
Answer:
Sulphur dioxide (SO2) acts as both oxidising as well as reducing agent.
SO2 as Oxidising agent:
Sodium suiphide oxidises to hypo with SO2.
2Na2S + 3SO2 → 2Na2S2O2 + S
SO2 as Reducing agent:
SO2 reduces Fe+3 ions to Fe+2 ions.
2Fe+3 + SO2 + 2H2O → 2Fe+2 + SO-24 + 4H+
![]()
Question 9.
Explain the conditions favourable for the formation of SO3 from SO2 in the contact process of H2SO4.
Answer:
Le Chatlier’s principle – Application to produce SO3:
The oxidation of SO2 to SO3 in the presence of a catalyst is a reversible reaction. The thermochemical equation for the conversion is written as
2SO2(g) + O2(g) ⇌ 2SO3(g); ∆H = -196 kJ
The equation reveals the following points :
- 3 volumes of the reactants convert into 2 volumes of SO3. i.e., a decrease of volume accompanies the reaction.
- the reaction is an exothermic change.
- the catalyst may be present to increase the SO3 yields.
According to Le Chatlier’s principle,
i) a decrease in volume of the system is favoured at high pressures. But in practice only about 2 bar pressure is used. The reason for not using high pressures is acid resisting towers which can withstand high pressures cannot be built.
ii) exothermic changes are favoured at low temperatures. It is not always convenient in the industry to work at low temperatures. In such situations an optimum temperature is maintained. At this temperature considerable amounts of the product are obtained. In the manufactrue of H2SO4, the optimum temperature suitable for the conversion of SO2 into SO3 is experimentally found to be 720K.
iii) The rate of formation of SO3 is enhanced by the use of a catalyst. (V2 O5 (or) Pt – asbestos).
Favourable Conditions :
Temperature : 720K
Pressure : 2 bar
Catalyst: V2O5 (or) platinized asbestos.
Question 10.
Complete the following
a) KCl + H2SO4 (Conc) →
b) Sucrose ![]()
c) Cu + H2SO4 (Conc) →
d) C + H2SO4 (Cone) →
Answer:
a) 2KCl + Cone. H2SO4 → 2HCl + K2SO4
b) C12H22O11 ![]() 12C + nH2O
12C + nH2O
c) Cu + 2H2SO4(Conc) → CuS04 + SO2 + 2H2O
d) C + 2H2SO4(Conc) → CO2 + 2SO2 + 2H2O
Question 11.
Which is used for drying ammonia ?
Answer:
For drying ammonia quick line (CaO) is used.
For drying ammonia cone. H2SO4, P4O10 and anhydrous CaCl2 cannot be used because they react with ammonia and forms (NH4)2SO4, (NH4)3PO4 and CaCl2. 8NH3
H2SO4 + 2NH3 → (NH4)2 SO4
![]()
CaCl2 + 8NH3 → CaCl38NH3.
![]()
Question 12.
Why cone H2SO4, P4O10 and anhydrous CaCl2 cannot be used to dry ammonia ?
(Hint: ammonia reacts with them forming (NH4)2 SO4; (NH4)3 PO4 and CaCl2, 8NH2)
Answer:
For drying ammonia quick line (CaO) is used.
For drying ammonia cone. H2SO4, P4O10 and anhydrous CaCl2 cannot be used because they react with ammonia and forms (NH4)2SO4, (NH4)3PO4 and CaCl2. 8NH3
H2SO4 + 2NH3 → (NH4)2 SO4
![]()
CaCl2 + 8NH3 → CaCl38NH3.
Long Answer Questions
Question 1.
Explain in detail the manufacture of sulphuric acid by contact process. [T.S. Mar. 18, 16]
Answer:
Manufacture of H2SO4 by contact process :
Manufacturing of H2SO4 involves three main steps.
Step – 1.
SO2 production : The required SO2 for this process is obtained by burning S(or) Iron pyrites in oxygen.
S + O2 → SO2
4FeS2 + 15O2 → 2Fe2O3 + 8SO3
Step – 2
SO3 formation: SO2 is oxidised in presence of catalyst with atmosphric air to form SO3
Le Chatlier’s principle – Application to produce SO3 :
The oxidation of SO2 to SO3 in the presence of a catalyst is a reversible reaction. The thermochemical equation for the conversion is written as
2SO2(g) + O2(g) ⇌ 2SO3(g); ∆H = -196 kJ
The equation reveals the following points : .
- 3 volumes of the reactants convert into 2 volumes of SO3. i.e., a decrease of volume accompanies the reaction.
- the reaction is an exothermic change.
- the catalyst may be present to increase the SO3 yields.
According to Le Chatlier’s principle, .
i) a decrease in volume of the system is favoured at high pressures. But in practice only about 2 bar pressure is used. The reason for not using high pressures is acid resisting towers which can withstand high pressures cannot be built.
ii) exothermic changes are favoured at low temperatures. It is not always convenient in the industry to work at low temperatures. In such situations an optimum temperature is maintained. At this temperature considerable amounts of the product are obtained. In the manufactrue of H2SO4, the optimum temperature suitable for the conversion of SO2 into SO., is experimentally found to be 720K.
iii) The rate of formation of SO3 is enhanced by the use of a catalyst. (V2 O5 (or) Pt – asbestos).
Favourable Conditions :
Temperature : 720K .
Pressure : 2 bar
Catalyst: V2O5 (or) platinized asbestos.
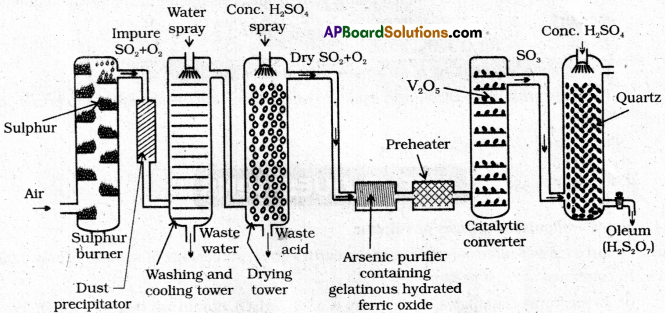
Step – 3 :
Formation of H2SO4: SO3 formed in the above step absorbed in 98% H2SO4 to get oleum (H2S2O7). This oleum is diluted to get desired concentration of H2SO4.
SO3 + H2SO4 → H2S2O7
H2S2O7 + H2O → 2H2SO4
![]()
Question 2.
How is ozone prepared from oxygen ? Explain its reaction with [A.P. Mar. 19, 18, 17, 16] [Mar. 14]
a) C2H4
b) KI
c) Hg
d) PbS.
Answer:
Preparation of Ozone :
A slow dry stream of oxygen under silent electric discharge to form ozone (about 10%). The product obtained is known as ozonised oxygen.
3O2 → 2O3; ∆H° = 142kJ/mole
- The formation of ozone is an endothermic reaction.
- It is necessary to use silent electric discharge in the preparation of O3 to prevent its decomposition
a) Reaction with C2H3 : Ethylene reacts with ozone to form Ethylene ozonoid followed by the hydrolysis to form formaldehyde.
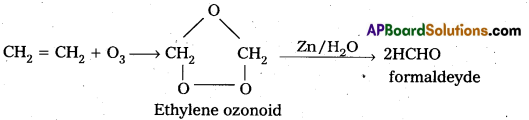
) Reaction with KI: moist KI is oxidised to Iodine in presence of ozone.
2KI + H2O + O3 → 2KOH + I2 + O2
c) Reaction with Hg : Mercury loses it’s lustreness, meniscus and consequently sticks to the walls of glass vessel when it reacts with ozone. This phenomenon is called tailing of mercury.
2Hg + O3 → Hg2O + O2
It is removed by shaking it with water which dissolves Hg2O.
d) Reaction with PbS : Black lead sulphide oxidised to white lead sulphate in presence of ozone.
PbS + 4O3 → PbSO4 + 4O2.
Intext Questions
Question 1.
List the important sources of sulphur.
Answer:
Occurrence or sources of sulphur in the earth’s crust, percentage of sulphur is only 0.03 to 0. 1%. In combined state, it occurs
- In the form of sulphates, eg., gypsum (CaSO4 . 2H2O). epsom salt (MgSO4 . 7H2O), baryte
(BaSO4). - In the form of sulphides, eg., galena (PbS), zinc blende (ZnS), Copper pyrites (CuFeS2). In volcanoes, traces of sulphur occur as H2S. In organic materials such as eggs, proteins, garlic, onion,mustard, hair and wool, sulphur is present in trace amounts.
Question 2.
Write the order of thermal stability of the hydrides of group 16 elements.
Answer:
The thermal stability of hydrides of group 16 elements is directly proportional to the bond dissociation enthalpy of H – E bond. On moving down the group, bond dissociation energy decreases because bond length increases.
Thus, the order of bond dissociation energy is
H2O > H2S > H2Se > H2Te > H2Po
This is also the order of thermal stability.
![]()
Question 3.
Why is H2O a liquid and H2S a gas ?
Answer:
The difference in electronegativity values of 0(3.5) and H(2.1) is more than the difference between the electronegativity values of H(2.1) and S(2.5) i.e., O – H bond is more polar than S – H bond. That is why H-bonding is present among water molecules but absent in H2S. Thus,
strong intermolecular interactions causes water to exist as a liquid but due to weak van der waals’ force H2S exists as a gas.
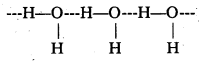
Question 4.
Which of the following does not react with oxygen directly ? Zn, Ti, Pt, Fe.
Answer:
Platinum (Pt).
Question 5.
Complete the following reactions.
i) C2H4 + O2 →
ii) 4Al + 3O2 →
Answer:
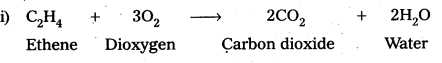
![]()
Question 6.
Why does O3 act as a powerful oxidising agent ?
Answer:
Because ozone liberates nascent oxygen very easily.
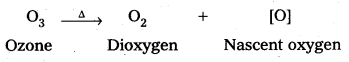
Question 7.
How is Oa estimated quantitatively ?
Answer:
When ozone reacts with an excess of potassium iodide solution buffered with a borate buffer (pH 9.2), iodine is liberated which can be titrated against a standard solution of sodium thiosulphate. In this way O3 can be estimated quantitatively.
Question 8.
What happens when sulphur dioxide is passed through an aqueous solution of Fe (III) salt ?
Answer:
When SO2 is passed through an aqueous solution of Fe (III), i.e., ferric salt, it is reduced to Fe (II) i,e., ferrous salt. Here, SO2 acts as a reducing agent.
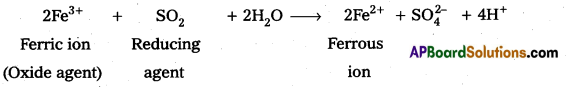
Question 9.
Comment on the nature of two S – O bonds formed in SO2 molecule. Are the two S – O bonds in this molecule equal ?
Answer:
The two S – O bonds in SO2 molecule are covalent in nature. These are equal with bond length = 143 pm. The resonating structures of SO2 are as follows.
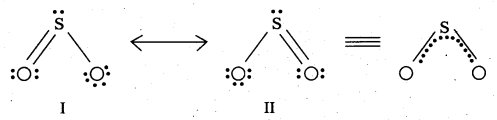
SO2 is a resonance hybrid of these two canonical forms.
![]()
Question 10.
How is the presence of SO2 detected ?
Answer:
SO2 has a pungent odour. Two tests to detect the presence of SO2 are as follows :
- SO2 decolourises acidified KMnO4 solution.
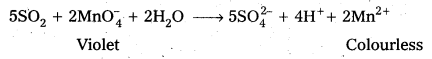
- SO2 Changes the colour of acidified potassium dichromate solution from orange to green.
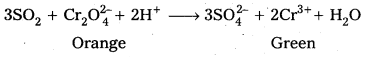
Question 11.
Mention three areas in which H2S04 plays an important role.
Answer:
Uses of sulphuric acid.
- It is used in the manufacture of pigments, paints and dyestuff intermediate.
- It is used in petroleum refining.
- It is also used in fertilizer industry.
![]()
Question 12.
Write the conditions to maximize the yield of H2SO4 by contact process.
Answer:
The key step in the manufacture of H2SO4 is catalytic oxidation of SO2 to produce SO3 in presence of V2O5.
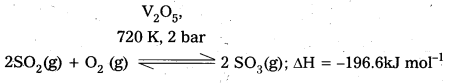
The reaction is exothermic, reversible and the forward reaction results in the decrease in volume. Thus according to Le-Chatelier’s principle, the forward reaction should be favoured by low temperature and high pressure. But the temperature should not be very low otherwise the. rate of reaction will become very slow.