Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material 10th Lesson Chemistry In Everyday Life Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material 10th Lesson Chemistry In Everyday Life
Very Short Answer Questions
Question 1.
What are drugs ?
Answer:
Drug : The chemicals of low molecular masses ranging from 100 to 500 U that react with macromolecular targets to produce biological response are called drugs.
E.g.: Morphine, Codeine, Heroin etc.,
Question 2.
When are the drugs called medicines ?
Answer:
When the biological response of a drug is therapeutic arid useful then the chemical substances (drugs) are called medicines.
![]()
Question 3.
Define the term chemotherapy.
Answer:
Chemotherapy : The use of medicines (chemical substances) in the treatment of diseases is called chemotherapy.
In chemotherapy diagnosis, prevention and treatment of diseases involved.
Question 4.
Name the macromolecules that are chosen as drug targets.
Answer:
The macromolecules that are chosen as drug targets are carbohydrates, lipids, proteins, nucleic acids and enzymes.
Question 5.
What are enzymes and receptors ?
Answer:
Enzymes : The proteins which perform the role of biological catalysts in the body are called enzymes.
Receptors : The proteins which are crucial to communication system in the body are called receptors.
Question 6.
Which forces are involved in holding the drug to the active site of enzymes ?
Answer:
The forces involved in holding the drugs to the active site of enzymes are ionic bonds, vander waal’s forces, hydrogen bonds, dipole-dipole interactions etc.,
![]()
Question 7.
What are enzyme inhibitors ?
Answer:
The drugs which inhibits the catalytic activity of enzymes and can block the binding site of the enzyme and prevent the binding of substrate are called enzyme inhibitors.
Question 8.
What is allosteric site ?
Answer:
Some drugs do not bind to the enzyme’s active site and bind to a different site of enzyme. This different site of enzyme is called allosteric site.
Question 9.
What are antagonists and agonists ?
Answer:
Antagonists : The drugs that bind to the receptor site and inhibit its natural function are called antagonists.
- These are useful when blocking of message is required.
Agonists : The drugs that mimic the natural messenger by switching on receptors are called agonists.
- These are useful when there is lack of natural chemical messenger.
Question 10.
Why do we need to classify the drugs in different ways ?
Answer:
- By knowing the pharmacological effect it makes easy for doctors.
- Antacid can be used in case of excessive acidity in stomach.
![]()
Question 11.
What are antacids ? Give example. [Mar. 14]
Answer:
Antacids : Chemicals that remove the excess of acid in the stomach and maintain the pH to normal level are antacids. E.g. : Omeprazole, Lansoprozole etc.,
Question 12.
What are antihistamines ? Give example.
Answer:
Antihistamines : Chemicals that prevent the interaction of histamines with receptors of the stomach wall thus producing less amount of acid are antihistamines.
E.g. : Dimetapp, Terfenadine (Seldane).
Question 13.
While antacids and antiallergic drugs interfere with the function of histamines why do not these interfere with the function of each other ?
Answer:
Antacids and antiallergic drugs donot interfere with the function of each other because they work on different receptors in the body.
Question 14.
What are tranquilizers ? Give example.
Answer:
Tranquilizers : The drugs which are used in the management (or) treatment of psychoes and neuroses are called tranquilizers.
E.g.: Luminal, Seconal, Barbituric acid etc.,
![]()
Question 15.
What are barbiturates ?
Answer:
Barbiturates : Derivatives of barbituric acid which functions as important class of tranquilizers are called barbiturates.
E.g.: Veronal, Amytatt etc.,
Question 16.
What are analgesics ? How are they classified ?
Answer:
Analgesics : These are to reduce or totally abolish pain without causing impairment of consciousness, mental confusion, incoordination, paralysis, disturbances of nervous system etc. Analgesics are classified as
- Narcotic analgesics : These are most potent and clinically useful agents causing depression of central nervous system and at the same time act as strong analgesics.
E.g. : Morphine, Codeine etc. - Non-narcotic analgesics : These drugs are analgesics but they have no addictive properties. Their analgesic use is limited to mild aches and pains.
E.g.: Aspirin, Ibuprofen etc.
Question 17.
What are narcotic analgesics ? Give example.
Answer:
Narcotic analgesics: These are most potent and clinically useful agents causing depression of central nervous system and at the same time act as strong analgesics.
E.g. : Morphine, Codeine etc.
Question 18.
What are non-narcotic analgesics ? Give example.
Answer:
Non-narcotic analgesics: These drugs are analgesics but they have no addictive properties. Their analgesic use is limited to mild aches and pains.
E.g.: Aspirin, Ibuprofen etc.
![]()
Question 19.
What are antimicrobials ?
Answer:
Antimicrobials : The chemical substances which destroy (or) prevent the developement (or) inhibit the pathogenic action of microbes such as bacteria, fungi, virus are called antimicrobials.
E.g.: Lysozyme, Lactic acid etc.,
Question 20.
What are antibiotics ? Give example. [T.S. Mar. 19; A.P. Mar. 16]
Answer:
Antibiotics: The chemical substances produced by micro organisms and inhibit the growth or destroy microorganisms are called antibiotics.
(Or)
The substance produced totally or partly by chemical synthesis which in low concentration inhibits the growth (or) destroy microorganism by intervening in their metabolic process are called antibiotics.
E.g. : Penicillin, Chloramphenicol etc.
Question 21.
What are antiseptics ? Give example. [T.S. Mar. 19; A.P. Mar. 19, 15]
Answer:
Antiseptics : The chemical compounds that kill (or) prevent the growth of micro organism are called antiseptics.
Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
E.g. : Dettol, Bithional etc.
Question 22.
What are disinfectants ? Give example.
Answer:
Disinfectants : The chemical compounds used for killing (or) preventing the growth of microorganism are called disinfectants.
- These are applied to inanimate objects like floors, drainage systems etc…
E.g.:- 4% aqueous solution of formaldehyde called formalin is a disinfectant
- 0.3 ppm chlorine aqueous solution is disinfectant.
- SO2 in very low concentration is disinfectant.
![]()
Question 23.
Name a substance which can be used as an antiseptic as well as disinfectant.
Answer:
Phenol is used as antiseptic as well as disinfectant.
- 0.2% phenol is antiseptic.
- 1% phenol is disinfectant.
Question 24.
What is the difference between antiseptics and disinfectants ?
Answer:
Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
Disinfectants are applied to inanimate objects such as floors, drainage system, instruments etc.
Question 25.
What are the main constituents of dettol ?
Answer:
Dettol (antiseptic) is a mixture of chloroxylenol and terpineol.
Question 26.
What is tincture of iodine ? What is its use ?
Answer:
Tincture of iodine (antiseptic) is a mixture of 2 – 3% Iodine solution in alcohol-water.
![]()
Question 27.
What are antifertility drugs ? Give example.
Answer:
Antifertility drugs: These are birth control pills and contain a mixture of synthetic estrogen and progesterone derivatives.
E.g. : Norethindrone, Ethynylestradiol (novestrol)
Question 28.
Why chemicals are added to food ?
Answer:
Chemicals are added to food for
- preservation
- enhancing their appeal
- adding nutritive value in them.
Question 29.
Name different categories of food additives.
Answer:
The following are the categories of food additives.
- Food colours
- Flavours and sweetners
- Fat Emulsifiers and stabilising agents
- Anti oxidants
- Flour improvers – antistaling agents and bleaches
- Preservatives .
- Nutritional supplements such as minerals, vitamins and amino acids.
Question 30.
What are artificial sweetening agents ? Give example. [A.P. Mar. 16; 15]
Answer:
The chemical substances which are used instead of sucrose (or) sugar are called artificial sweetening agents.
E.g.: Aspartame, Alitame, saccharin.
These decrease the calorific intake and at the same time several times sweater than sucrose.
![]()
Question 31.
Why do we require artificial sweetening agents ?
Answer:
- Artificial sweetening agents are very useful to diabetic persons.
- These decrease the calorific in take and at the same time several times sweeter than sucrose.
- These are harmless.
Question 32.
Why is the use of aspartame limited to cold foods and drinks ?
Answer:
Aspartame is unstable at cooking temperature so it’s use is limited to cold foods and soft drinks. –
Question 33.
Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Answer:
The sweetening agent used in the preparation of sweets for a diabetic patient is saccharin (or) sucralose. It is stable at cooking temperature.
Question 34.
What problem does arise in using alitame as artificial sweetener ?
Answer:
While using alitame as artificial sweetener, the control of sweetness of food is difficult. Alitame is a high potency sweetner.
Question 35.
What are food preservatives ? Give example.
Answer:
The chemical substances which prevent the spoilage of food due to microbial growth are called food preservatives.
E.g.: Sodium benzoate, Salt of sorbic acid etc.
![]()
Questions 36.
Name two most familiar antioxidants used as food additives.
Answer:
The most familiar antioxidants are butylated hydroxy toluene (BHT) and butylated hydroxy anisole (BHA)
Question 37.
What is saponification ?
Answer:
The process of formation of soaps Containing sodium salts by heating esters of fatty acid with aq. NaOH solution is called saponification.
Questions 38.
What are soaps chemically ?
Answer:
Chemically soaps are sodium (OF) potassium salts of long chain fatty acids.
E.g.: Sodium stearate.
Question 39.
Why do soaps not work in hard water ?
Answer:
In hard water Ca, Mg-dissolved salts are present. Ca+2, Mg+2 ions form insoluble Ca, Mg, soaps respectively when sodium (or) potassium soaps are dissolved in hard water.
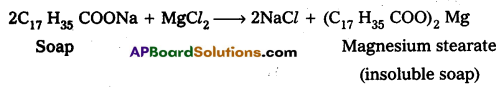
- These insoluble soaps separate as scum in water and are useless as cleansing agent. These are problematic to good washing because the ppt adheres into the fibres of cloth as gummy mass.
- Hair washed with hard water looks dull.
- Dye does not absorb evenly on cloth washed with soap using hard water due to this gummy mass.
![]()
Question 40.
What are synthetic detergents ?
Answer:
The cleasing agents which are having all the properties of soaps but do not contain any soap are called synthetic detergents.
Synthetic detergents can be used both in soft and hard water as they give foam even in hard water.
E.g.: Sodium dodecyl benzene sulphonate.
Question 41.
What is the difference between a soap and a synthetic detergent ?
Answer:
- Generally soaps are sodium or potassium salts of long chain fattyacids.
- Synthetic detergents are cleansing agents having all the properties of soaps and do not contain any soap.
- Soaps do not work in hard water but synthetic detergents can be used both in soft and hard water as they give foam even in hard water. Some of the detergents give foam even in ice cold water.
Question 42.
How are synthetic detergents better than soaps ? [Mar 14]
Answer:
Soaps do not work in hard water but synthetic detergents can be used both in soft and hard water as they give foam even in hard water. Some of the detergents give foam even in ice cold water.
Question 43.
Name the different categories of synthetic detergents.
Answer:
Synthetic detergents are classified into three types
- nionic detergents
- Cationic detergents
- Non-ionic detergents.
Question 44.
Can you use soaps and synthetic detergents to check the hardness of water ?
Answer:
- Soaps are used to check the hardness of water because soaps form insoluble precipitate with hard water.
- Detergents are soluble in both types of water i.e hardwater and soft water, so detergents are not used to check the hardness of water.
![]()
Question 45.
If water contains dissolved calcium hydrogen carbonate, out of soaps and synthetic detergents which one will you use for cleaning clothes and why ?
Answer:
Water contains dissolved calcium hydrogen carbonate is called hard water. This water form insoluble precipitate with soap. Synthetic detergents donot form this type of precipitates, so synthetic detergents are used for cleaning clothes with water containing dissolved Ca(HCO3)2.
Short Answer Questions
Question 1.
Explain the term target molecules or drug targets as used in medicinal chemistry.
Answer:
Drug Targets (or) Target molecules: Macro molecules like carbohydrates, lipids, proteins, nucleic acids and enzymes which interact with the drugs are ailed drug targets (or) Target molecules.
Question 2.
Explain the catalytic action of enzymes as drug targets.
Answer:
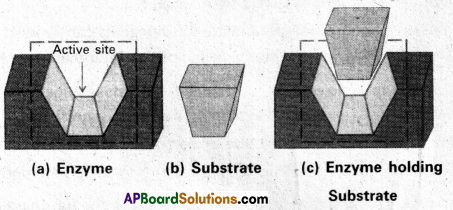
a) Catalytic action of Enzymes : In the enzyme catalytic activity it performs two functions
- The first function of an enzyme is to hold the substrate for a chemical reaction. Substrate bind to active site of enzyme through ionic bonding, hydrogen bonding, vander waal force etc.
- The second function of an enzyme is to provide functional groups that will attack the substrate, and carry out the chemical reaction.
b) Drug- Enzyme interaction :
- Drugs which inhibit the catalytic activity of the enzymes are called Enzyme inhibitors.
- Drugs which compete with the substrate for their attachment on active sites of enzymes are called competitive inhibitors.
![]()
Question 3.
Explain the drug – enzyme interaction.
Answer:
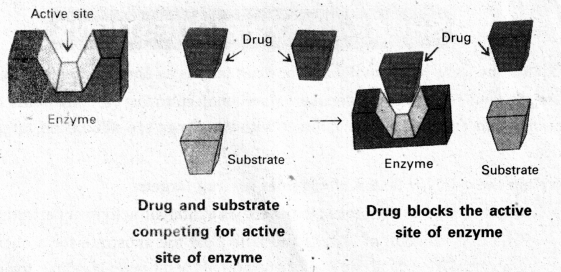
Explanation of drug – Target interaction with Enzymes :
- The proteins which perform the role of biological catalysts in the body are called enzymes.
- The binding of inhibitor ai allosteric site changes the shape of the active site.
- If the bond formed between an enzyme and an inhibitor is a strong covalent bond it cannot be broken easily In this case enzyme is blocked permanently and the body then degrades the enzyme-inhibitoi complex and synthesises the new enzyme.
Question 4.
Why are cimetidine and ranitidine better antacids than sodium hydrogen carbonate or magnesium hydroxide or aluminium hydroxide.
Answer:
Sodium hydrogen carbonate or magnesium hydroxide (or) aluminium hydroxide treatment as antacids control only symptoms and not the cause. In advanced stages, ulcers become life threatening and only treatment is removal of affected part of stomach.
The drugs cinetidine, ranitidine prevent the interaction of histamine with the receptors present in the stomach wall and results in release of lesser amount of acid.
So cinetidine, ranitidine are better antacids than NaHCO3 (or) Mg(OH)2 (or) Al(OH)3.
Question 5.
Low level of noradrenaline in the cause of depression. What type of drugs are needed to cure this problem ? Name two drugs.
Answer:
If the level of Noradrenaline is low then the signal sending activity becomes low and the person suffers from depression. In this situations antidepressant drugs (tranquilizers) are required, These drugs inhibit the enzymes which catalyse the degradation of noradrenaline. If the enzyme is inhibited, this important neurotransmitter is slowly metabolised and can activate its receptor for longer periods of time.
Two important antidepressant drugs are IProniazid and phenelzine.
![]()
Question 6.
What are analgesics ? How are they classified ? Give examples. [T.S. & A.P. Mar. 19]
Answer:
Analgesics : These are to reduce or totally abolish pain without causing impairment of consciousness, mental confusion, incoordination, paralysis, disturbances of nervous system etc.
Analgesics are classified as
- Narcotic analgesics : These are most potent and clinically useful agents causing depression of central nervous system and at the same time act as strong analgesics.
E.g. : Morphine, Codeine etc. - Non-narcotic analgesics : These drugs are analgesics but they have no addictive properties. Their analgesic use is limited to mild aches and pains.
E.g.: Aspirin, Ibuprofen etc.
Question 7.
What are different types of microbial drugs ? Give one example for each.
Answer:
Antimicrobials : The chemical substances which destroy or prevent the developement (or) inhibit the pathogenic action of microbes such as bacteria, fungi, virus are called antimicrobials.
E.g.: Lysozyme, Lactic acid etc.,
Different types of antimicrobial drugs are antibiotics, antiseptics, disinfectants.
1) Antibiotics : The chemical substances produced by micro organisms and inhibit the growth or destroy microorganisms are called antibiotics.
(Or)
The substance produced totally or partly by chemical synthesis in low concentration inhibits the growth (or) destroy microorganism by intervening in their metabolic process are called antibiotics.
2) Antiseptics: The chemical compounds that kill (or) prevent the growth of micro organism
are caiied antiseptics. .
Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
3) Disinfectants : The chemical compounds used for killing (or) preventing the growth of microorganism are called disinfectants.
These are applied to inanimate objects like floors, drainage systems etc…
E.g.:
- 4% aqueous solution of formaldehyde called formalin is a disinfectant
- 0.3 ppm chlorine aqueous solution is disinfectant.
- SO2 in very low concentration is disinfectant.
![]()
Question 8.
Write the characteristic properties of antibiotics.
Answer:
Characteristic properties of antibiotics :
- Antibiotic drugs must be the products of metabolism. ,
- Antibiotic drugs are effective in low concentration.
- Antibiotic drug retards the growth (or) survival of microorganism.
- Antibiotic should be a synthetic substance produced as a structural analogue of naturally occurring antibiotic.
- Antibiotics have either cidal (killing) effect (or) a static (inhibiting) effect on microbes.
E.g.: Penicillin, Ofloxacin are Bactericidal.
Erythromycin, Tetracycline are Bacteriostatic.
Question 9.
What is meant by the term broad spectrum antibiotics ? Explain.
Answer:
The range of bacteria (or) other micro organisms that are effected by a certain antibiotic is expressed as its spectrum of action.
Broad spectrum antibiotics : Antibiotics which kill (or) inhibit a wide range of gram-positive and gram-negative bacteria are called broad spectrum antibiotics.
Question 10.
What are broad spectrum and narrow spectrum antibiotics ? Give one example for each.
Answer:
The range of bacteria (or) other micro organisms that are effected by a certain antibiotic is expressed as its spectrum of action.
Broad spectrum antibiotics : Antibiotics which kill (or) inhibit a wide range of gram¬positive and gram-negative bacteria are called broad spectrum antibiotics.
Narrow spectrum antibiotics: Antibiotics which are effective mainly against gram-positive (or) gram-negative bacteria are called narrow spectrum antibiotics,
E.g.: Penicillin – G is a narrow spectrum antibiotic.
Limited spectrum antibiotics : Antibiotics which are effective mainly against a single organism (or) disease are called as limited spectrum antibiotics.
![]()
Question 11.
Write notes on antiseptics and disinfectants.
Answer:
Antiseptics: The chemical compounds that kill (or) prevent the growth of micro organism are called antiseptics.
Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
E.g.: Dettol, Bithional etc.
Phenol is used as antiseptic as well as disinfectant. 0.2% phenol is antiseptic.
Dettol (antiseptic) is a mixture of chloroxylenol and terpineol.
Tincture of iodine (antiseptic) is a mixture of 2 – 3% Iodine solution in alcohol-water.
Disinfectants : The chemical compounds used for killing (or) preventing the growth of microorganism are called disinfectants.
- These are applied to inanimate objects like floors, drainage systems etc…
E.g.: 4% aqueous solution of formaldehyde called formalin is a disinfectant. - 0.3 ppm chlorine aqueous solution is disinfectant.
- SO2 in very low concentration is disinfectant.
Phenol is used as antiseptic as well as disinfectant 1% phenol is disinfectant.
Question 12.
How do antiseptics differ from disinfectants ? Does the same substance be used as both ? Give one example for each.
Answer:
Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
Disinfectants are applied to inanimate objects such as floors, drainage system, instruments etc.
Phenol is used as antiseptic as well as disinfectant.
i) 0.2% phenol is antiseptic.
ii) 1% phenol is disinfectant.
- Examples of Antiseptics :
- Dettol (antiseptic) is a mixture of chloroxylenol and terpineol.
- Tincture of iodine (antiseptic) is a mixture of 2.3% Iodine solution in alcohol-water.
- Examples of disinfectants :
- 4% aqueous solution of formaldehyde called formalin is a disinfectant.
- 0.3 ppm chlorine aqueous solution is disinfectant.
- SO2 in very low concentration is disinfectant.
Phenol is used as anti in very low concentration is disinfectant.
![]()
Question 13.
What are the main categories of food additives ?
Answer:
The following are the categories of food additives.
- Food colours
- Flavours and sweetners
- Fat Emulsifiers and stabilising agents
- Anti oxidants
- Flour improvers – antistaling agents and bleaches
- Preservatives
- Nutritional supplements such as minerals, vitamins and amino acids.
Question 14.
Write notes on antioxidants in food.
Answer:
Antioxidants :
- Antioxidants are important and necessary food additives.
- Antioxidants help in food preservation by retarding the action of oxygen on food. Antioxidants are more reactive towards, oxygen than the food material which they protecting.
- The most familiar antioxidants are Butylated hydroxy toluene (BHT) and Butylated hydroxy anisole (BHA).
- The addition of BHA to butter increases its shelf life from months to years.
- BHT and BHA along with citric acid are added to produce more antioxidant effect.
- SO2 and sulphites are useful anti Oxidants for wine and beer, sugar syrups and cut, peeled (or) dried fruits and vegetables.
Question 15.
Name different types of soaps.
Answer:
The following are the different types of soaps.
- Toilet soaps
- Soaps that float in water
- Medicated soaps
- Shaving soaps
- Laundry soaps
- Soap powders and scouring soaps etc.
![]()
Question 16.
Explain the following terms with suitable examples.
i) Cationic detergents
ii) Anionic detergents
iii) Non-ionic detergents
Answer:
Synthetic detergents are classified into three types.
i) Cationic detergents : These are synthetic detergents.
a) Cationic detergents are quarternary ammonium salts of amines with acetates, chlorides (or) bromides as anions.
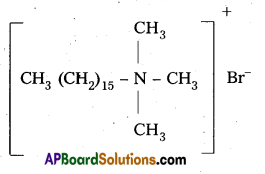
b) Cationic part possess a long hydrocarbon chain and a positive charge on nitrogen atom. Hence these are called catiomc detergents.
E.g. : Cetyl trimethyl ammonium bromide
It is used in hair conditioners.
ii) Anionic Detergents: These are synthetic detergents.
a) Anionic detergents are sodium salts of sulphonated long chain alcohols (or) hydrocarbons.
b) Anionic detergents are formed by the treatment of long chain alcohols with conc. H2SO4 followed by the neutralisation with alkali.
E.g.: Sodium lauryl sulphate.
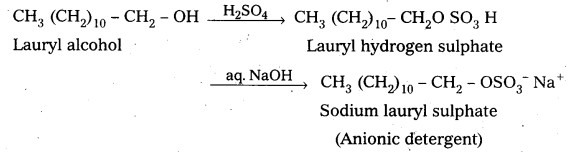
These are used for house hold work and in tooth pastes.
iii) Non-ionic detergents : These are synthetic detergents.
a) Non-ionic detergents do not contain any ion in their constitution.
b) The detergent formed by the reaction of stearic acid with poly ethylene glycol is an example of non ionic detergent.
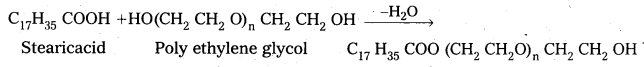
Non-ionic detergents are used in liquid dish washing purpose.
Question 17.
What are biodegradable and non-bio degradable detergents ? Give one example for each.
Answer:
- Biodegradable detergents :
The detergents which are degraded (or) decomposed by micro organisms are called biodegradable detergents. Biodegradable detergents have less branching.
These do not cause water pollution.
E.g.: n-dodecyle benzene sulphonate, soap (non synthetic), - Non Biodegradable detergents :
The detergents which are not decomposed (or) degraded by microbes (or) micro organisms are called non-biodegradable detergents. These have more branching.
These cause water pollution.
E.g.: ABS detergent.
![]()
Question 18.
Explain the cleansing action of soaps.
Answer:
Soap is sodium stearate, C17H35COONa, in water soap gives the ions stearate anion and sodium ion.
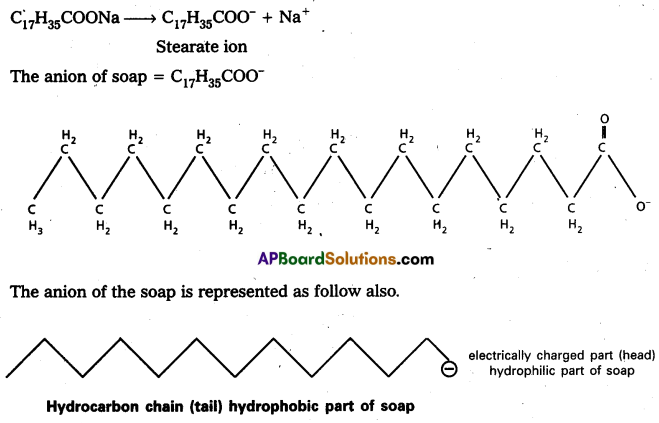
Cleansing action of soap : Soap anions form a micelle. The grease or dirt of the cloth are absorbed into the interior of the micelle. The tails of the anion are pegged into micelle and these micelle are washed away with the soap solution.

The main function of the soap is therefore, to convert the oily and greasy dirt on the cloth into large colloidal particles. For this a critical concentration of soap solution is required. Soap thus, functions as an emulsifying agent for the water – dirt emulsion. The emulsified grease or dirt is then washed away with soap solution.
Question 19.
Label the hydrophilic and. hydrophobic parts in the following compounds.
i) CH3 (CH2)10 CH2 OSO–3 Na+
ii) CH3(CH2)15N+(CH3)3 Br–
iii) CH3 (CH2)16 COO (CH2 CH2O)n CH2 CH2 OH
Answer:
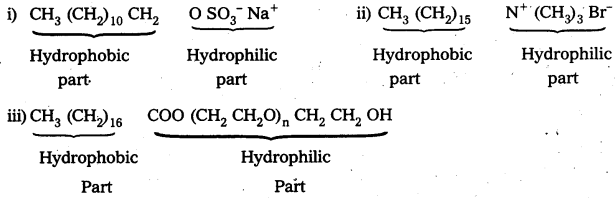
![]()
Question 20.
Draw the structures of following:
i) Serotonin
ii) Bithionol
iii) Chloramphenicol
iv) saccharin
Answer:
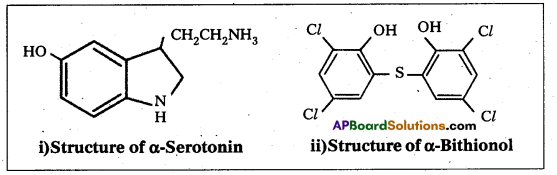
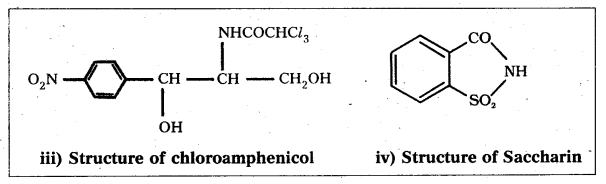
Long Answer Questions
Question 1.
Describe the classification of drugs into different classes.
Answer:
Classification of drugs : Drugs are classified in different ways.
- On the basis of drug action : Based on the actions of drugs on particular biochemical processes they are classified as
- Antibacterials
- Antibiotics
- Hypnotics
- Sedatives and tranquilizers
- Cardio vascular drugs
- Antiseptics etc.
- On the basis of molecular targets : Biomolecules like Carbohydrates, lipids, proteins, nucleic acids etc. react with drugs. These biomolecules are called drug targets or simply target molecules.
- On the basis of chemical structure : The drugs with some common structural features generally show similar pharmacological activity.
E.g.: Sulfonamides have common structural feature.
So they have same chemotherapeutic action. - On the basis of pharmocological effect:
This type of classification is useful for doctors because it provides them the whole range of drugs available for the treatment.
E.g.: Analgesics have pair killing effect.
![]()
Question 2.
Describe briefly the therapeutic action of different classes of drugs.
Answer:
The therapeutic action of different classes of drugs discussed below.
Antacids : Chemicals that remove the excess of acid in the stomach and maintain the pH to normal level are antacids.
E.g. : Omeprazole, Lansoprazole etc.,
Antihistamines : Chemicals that prevent the interaction of histamines with receptors of the stomach wall thus producing less amount of acid are anti histamines.
E.g.: Dimetapp, Terfenadine (Seldane)
Tranquilizers : The drugs which are used in the management (or) treatment of psychoes and neuroses are called tranquilizers
E.g.: Luminal, Seconal, Barbituric acid etc.,
Barbiturates : Derivatives of barbituric acid which functions as important class of tranquilizers are called barbiturates
E.g.: Veronal, Amytal etc.,
Analgesics : These are to reduce or totally abolish pain without causing impairment of consciousness, mental confusion, incoordination, paralysis, disturbances of nervous system etc.
Analgesics are classified as
- Narcotic analgesics : These are most potent and clinically useful agents causing depression of central nervous system and at the same time act as strong analgesics.
E.g.: Morphine, Codeine etc. - Non-narcotic analgesics : These drugs are analgesics but they have no addictive properties. Their analgesic use is limited to mild aches and pains.
E.g. : Aspirin, Ibuprofen etc.
Antimicrobials : The chemical substances which destroy (or) prevent the developement (or) inhibit the pathogenic action of microbes such as bacteria, fungi, virus are called antimicrobials. E.g.: Lysozyme, Lactic acid etc.,
Antibiotics: The chemical substances produced by micro Organisms and inhibit the growth or destroy microorganisms are called antibiotics. .
(Or)
The substance produced totally or partly by chemical synthesis in low concentration inhibits the growth (or) destroy microorganism by intervening in their metabolic process are called antibiotics.
E.g. : Penicillin, Chloramphenicol etc.
Antiseptics : The chemical compounds that kill (or) prevent the growth of micro organism are called antiseptics.
Antiseptics are the chemical substances applied on the living tissues such as wounds, cuts, ulcers and diseased skin surfaces.
Disinfectants : The chemical compounds used for killing (or) preventing the growth of microorganism are called disinfectants.
These are applied to inanimate objects like floors, drainage systems etc.
E.g.:
- 4% aqueous solution of formaldehyde called formalin is a disinfectant.
- 0.3 ppm chlorine aqueous solution is disinfectant.
- SO2 in very low concentration is disinfectant.
Antifertility drugs: These are birth control pills and contains a mixture of synthetic estrogen and progesterone derivatives.
E.g. : Norethindrone, Ethynylestradiol (novestrol).
Question 3.
Write an essay on antimicrobials.
Answer:
Antimicrobials : The chemical substances which destroy or prevent the developement (or) inhibit the pathogenic action of microbes such as bacteria, fungi, virus are called antimicrobials
E.g. : Lysozyme, Lactic acid etc.,
Different types of anti microbial drugs are antibiotics, antiseptics, disinfectants.
Question 4.
Write notes on the following : [T.S. Mar. 16]
i) Artificial sweetening agents
ii) Food preservatives
iii) Antioxidants in food.
Answer:
i) Artificial sweetening agents: The chemical substances which are used instead of sucrose (or) sugar are called artificial sweetening agents.
E.g.: Aspartame, Alitame, saccharin.
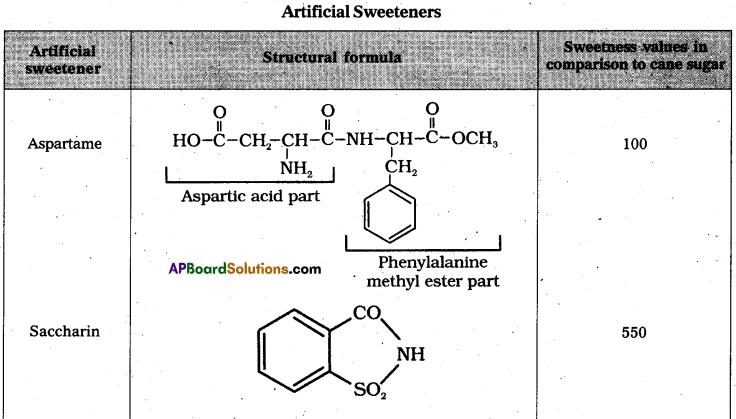
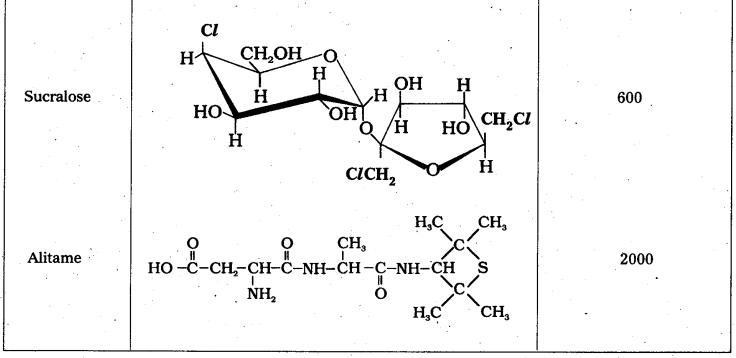
- These decrease the calorific intake and at the same time several times sweeter than sucrose.
- Artificial sweetening agents are very useful to diabetic persons.
- These are harmless.
- Aspartame is unstable at cooking temperature so it’s use is limited to cold foods and soft drinks.
- The sweetening agent used in the preparation of sweets for a diabetic patient is saccharin (or) sucralose. It is stable at cooking tenperature.
- While using alitame as artificial sweetener, the control of sweetness of food is difficult. Alitame is a high potency sweetner.
ii) Food preservatives : The chemical substances which prevent the spoilage of food due to microbial growth are called food preservatives.
Sodium benzoate, C6H5COONa is the most important food preservative used in limited quantities and is metabolised by conversion into hippuricacid. Which is finally excreted in the urine. Salts of sorbicacid, propionic acid are also food presevatives.
iii) Antioxidants :
- Antioxidants are important and necessary food additives.
- Antioxidants help in food preservation by retarding the action of oxygen on food.
- Antioxidants are more reactive towards oxygen than the food material which they protecting.
- The most familiar anti oxidants are Butylated hydroxy toluene (BHT) and Butylated hydroxy anisole (BHA).
- The addition of BHA to butter increases its shelf life from months to years.
- BHT and BHA along with citric acid are added to produce more antioxidant effect.
- SO2 and sulphits are useful anti oxidants for wine and beer, sugar syrups and cut, peeled (or) dried fruits and vegetables.
![]()
Question 5.
Write notes on the following :
i) Soaps
ii) Synthetic detergents.
Answer:
i) Soaps : 1) Soaps are the detergents which improve cleansing properties of water and help in removal of fats which bind other materials to the fabric (or) skin.
Chemically soaps are sodium (or) potassium salts of long chain fatty acids.
E.g.: Sodium stearate.
The process of formation of soaps containing sodium salts by heating esters of fatty acid with aq. NaOH solution is called saponification.
Only sodium and potassium soaps are soluble in water and are used for cleaning purpose. Potassium soaps are soft to the skin and are prepared by using KOH instead of NaOH.
Types of soaps :
The following are the different types of soaps
- Toilet soaps
- Soaps that float in water
- Medicated soaps
- Shaving soaps
- Laundry soaps –
- Soap powders and scouring soaps etc.
In hard water Ca, Mg-dissolved salts are present. Ca+2, Mg+2 ions form insoluble Ca, Mg, soaps respectively when sodium (or) potassium soaps are dissolved in hard water.
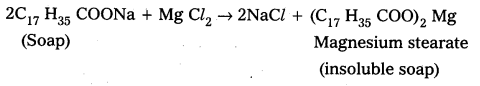
- These insoluble soaps separate as scum in water and are useless as cleansing agent. These are problematic to good washing because the ppt adheres into the fibres of cloth as gummy mass.
- Hair washed with hard water looks dull.
- Dye does not absorb evenly on cloth washed with soap using hard water due to this gummy mass.
ii) Synthetic detergents : The cleansing agents which are having all the properties of soaps but donot contain any soap are called synthetic detergents.
Synthetic detergents can be used both is soft and hardwater as they give foam even in hard water.
E.g. : Sodium dodecyl benzene sulphonate.
Soaps do not work in hard water but synthetic detegents can be used both in soft and hard water as they give foam even in hard water. Some of the detergents give foam even in ice cold water.
Synthetic Detergents are classified into three types :
i) Cationic detergents : These are synthetic detergents.
a) Cationic detergents are quarternary ammonium salts of amines with acetates, chlorides (or) bromides as anions.
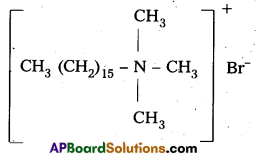
b) Cationic part possess a long hydrocarbon chain and a positive charge on nitrogen atom. Hence these are called cationic detergents.
E.g. : Cetyl trimethyl ammonium bromide.
It is used in hair conditioners.
ii) Anionic Detergents :
These are synthetic detergents.
a) Anionic detergents are sodium salts of sulphonated long chain alcohols (or) hydro-carbons.
b) Anionic detergents are formed by the treatment of long chain alcohols with cone, H2S04 followed by the neutralisation with alkali.
E.g. : Sodium lauryl sulphate.
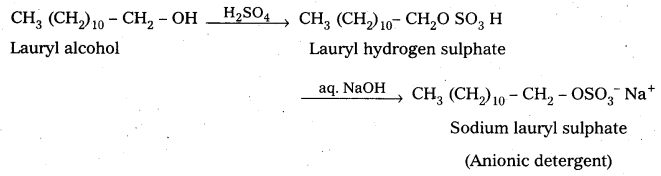
These are used for house hold work and in tooth pastes.
![]()
iii) Non-ionic detergents : .
These are synthetic detergents.
a) Non-ionic detergents donot contain any ion in their constitution.
b) The detergent formed by the reaction of stearic acid with poly ethylene glycol is an example of non ionic detergent.
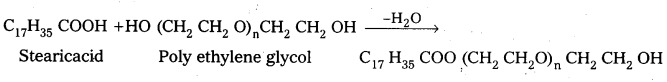
Non-ionic detergents are used in liquid dish washing purpose.
Intext Questions
Question 1.
Sleeping pills are recommended by doctors to the patients suffering from sleeplessness but it is not advisable to take its doses without consultation with the doctor. Why ?
• Consider the effect of tranquilizers or antidepressants on the nervous system.
Solution:
Sleeping pills contain drugs that may be tranquilizers or antidepressants. They affect the nervous system, relieve anxiety, stress, irritability or excitement. But they should strictly be used under the supervision of a doctor. If not, the uncontrolled and over dosage can cause harm to the body and mind because in higher doses these drugs act as poisons.
Question 2.
With reference to which classification has the statement, “ranitidine is an antacid” been given ?
Solution:
This statement refers to the classification of drugs according to pharmacological effect because in neutralizes the acidity (excess) of stomach.
Question 3.
Why do we require artificial sweetening agents ?
• Mention the function of natural sweeteners, discuss the same for artificial sweeteners.
Solution:
Natural sweeteners (sucrose etc.) provide calories to the body. Taking extra calories is harmful
for diabetic patients. So, artificial sweeteners are used
(a) to control intake of calories and
(b) as a substitute of sugar for diabetics.
![]()
Question 4.
Write the chemical equation for preparing sodium soap from glyceryl oleate and glyceryl palmitate. Structural formulae of these compounds are given below.
i) (C15H31COO)3 C3H5 – Glyceryl palmitate
ii) (C17H32COO)3 C3H5 – Glyceryl oleate
• Consider the following method.
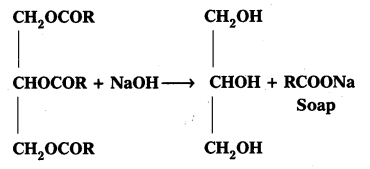
Solution:
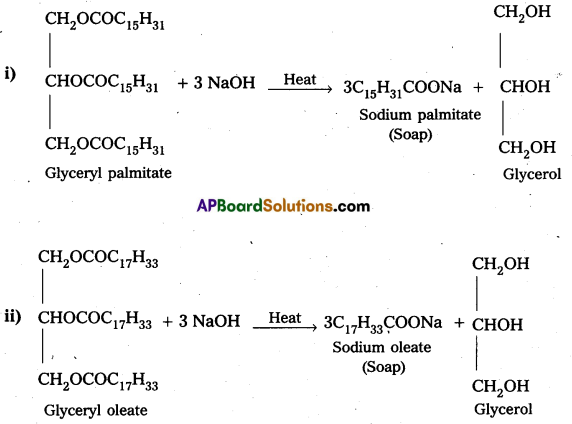
Question 5.
Following types of non-ionic detergents are present in liquid detergents, emulsifying agents and wetting agents. Label the hydrophilic and hydrophobic parts in the molecule. Identify the functional group (s) present in the molecule.
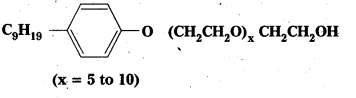
Solution:
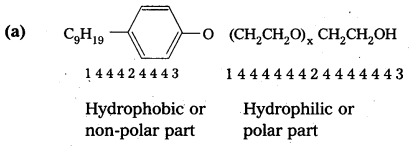
(b) Functional group : Ether and alcohol.