AP State Syllabus AP Board 8th Class Physical Science Solutions Chapter 8 Combustion, Fuels and Flame Textbook Questions and Answers.
AP State Syllabus 8th Class Physical Science Solutions 8th Lesson Combustion, Fuels and Flame
8th Class Physical Science 8th Lesson Combustion, Fuels and Flame Textbook Questions and Answers
Improve Your Learning
Question 1.
Give four examples of combustible materials.
Answer:
- Petrol
- Diesel
- Kerosene
- Alcohol
Question 2.
Give four examples of non-combustible materials.
Answer:
- Sand
- Clay
- Iron
- Steel
![]()
Question 3.
Why should not we store spirit or petrol near our living place?
Answer:
Spirit or petrol has low ignition temperature. So they turn into gas at room tempera¬ture. So they are highly inflammable that means they easily catch fire at room temperature. So in order to avoid fire accidents we should not store spirit or petrol near our living place.
Question 4.
Give an example of a good fuel. How do you choose that fuel? Explain.
Answer:
The example for good fuel is CNG.
The characteristics of good fuel.
- It should be cheap.
- It should be readily available.
- It should be readily combustible.
- It should be transported easily.
- It should have high calorific value.
- It should not produce gases or residue that pollute the environment.
CNG possess all these characteristics so it is an example for good fuel or ideal fuel.
Question 5.
The oil tires should not be sprayed with water. Why?
Answer:
We should not spray water on oil fires because water is heavier than the oil, it sinks below the oil and oil keeps burning on the top. So water is not suitable for oil fire accidents.
![]()
Question 6.
What precautions are to be taken while pouring water on fire?
Answer:
- Put off the electric mains.
- Do not pour water on fires caused by electricity water containing dissolves salts is a good conductor of electricity.
- Do not pour water on fires due to petrol and oil because water is more denser the u oil and petrol.
Question 7.
Why a wick is not used in gas burners ?
Answer:
Wax in the candle melts when it is lighted by a match and a little wax forms vapour. This vapour combines with oxygen in the air to form flame. The heat of the flame melts more of the wax from the top of the candle. The melted liquid wax move upward through the thread. It also changes to vapour when it reaches the top the wick and burns w’ h the flame.
Wick is not required in gas burners because LPG has low ignition temperature so it is easily convert into vapour state.
Question 8.
Water is not used to control fires involving electrical equipment. Why?
Answer:
Water is not used to control fires involving electric equipments because water is good
conductor of electricity (water having dissolved salts) so it may conduct electricity and harm those trying to douse the fire.
![]()
Question 9.
Give supporting arguments for both the statements (1) fire is useful (2) fire is harmful.
Answer:
Fire is useful:
- Fire is useful in cooking food.
- Fire is used in preparing different types of jewellery with gold and silver.
- Fire is utilized in making different types of articles with metals and plastics.
- The heat energy produced from fire of coal used to produce electricity.
Fire is harmful:
- During fire accidents fire can burn human organs and tissues causing severe damage to mankind, sometimes the people may die due to severe burns.
- During fire accidents the fire not only kill people but lot of damage to articles, equipments in the house.
- Fire can burn the forests in summer which may be harmful to animals present in the forest.
Question 10.
What would happen if oxygen stops to support combustion? – Make a guess. And if it is the situation for what other works fuels are useful?
Answer:
If oxygen stops to support combustion there is no other gas which will support combustion. Then fossil fuels are not useful in producing heat, energy and electricity.
So we should have to prefer alternative sources of energy like solar energy, wind energy, tidal energy, biomass energy, geothermal energy, etc. for our energy needs.
Question 11.
Let us assume that you are on the moon. If you try to focus sunlight on a paper using magnifying glass, does the paper catch fire? or not? Why?
Answer:
No, moon reflect entire sunlight falls on the surface because it acts as perfect reflector. Whereas earth is also acts as reflector but green house gases present in atmosphere absorbing the sunlight and resending on earth. So paper can be burnt on earth by using magnifying glass but it is not possible on moon.
![]()
Question 12.
Can you heat water in a paper vessel? How is it possible?
Answer:
In order to find the answer we have to do an experiment.
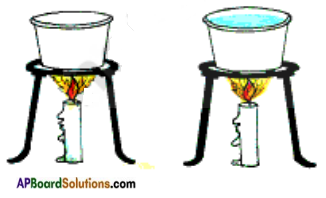
Take two small paper cups. Pour water in one of the cups. Put the two cups on different tripod stands and heat both of them using a candle shown in the figure. One cup burns quickly and other does not.
The reason is when heat is supplied to cups, the heat received by second cup is transferred to water
in it. The water in this cup prevents the paper to reach its ignition temperature and hence it does not burn. So water can be heated in a paper vessel upto ignition temperature of paper.
Question 13.
“Is combustion possible without the supply of oxygen”? Discuss with your teacher.
Answer:
No, combustion is not possible without oxygen because it is a reaction of material with oxygen that is an oxidation process. For example, carbon compounds react with oxygen and form carbon-dioxide and water. So no combustion reaction is possible without oxygen.
Question 14.
Explain giving reasons: In which of the following situations water will get heated in a shorter time?
a) Srikar kept water beaker near the wick in the yellow part of a candle flame.
b) Sonu kept water beaker in the outermost part of the flame.
Answer:
In the second situation water will get heated in a short time in the outermost part in the flame complete combustion takes place so which is hottest part whereas yellow part is in the middle zone of candle where partial combustion takes place and it is moderately hot.
Question 15.
List the ways adopted by fire fighters to combat fires.
Answer:
Ways adopted by fire fighters:
- The fire fighters immediately put off the electric mains and start spraying water on the fire. The water spray cool the combustible material below the ignition tempera¬ture. This prevents fire from spreading. Then the heat turns the water into vapours which surround the burning material and prevent supply of oxygen to the burning material. So the fire extinguishes.
- For electrical fire accidents or fire accidents involving oil and petrol water is not useful. So fire fighters use carbondioxide. It is heavier than oxygen and does not affect the electric equipment.
Question 16.
Collect information available on different fuels. Find out the cost per kg and compare the cost with calorific value. Prepare report on that.
Answer:
| Fuel | Cost per kg (or) litre | Calorific value |
| 1. LPG | Rs. 67 | 55000 |
| 2. Diesel | Rs. 58 | 45000 |
| 3. Petrol | Rs. 80 | 45000 |
| 4. CNG | Rs. 49 | 50000 |
From the table we will observe CNG has least price and also comparatively equal calorific value with other fuels and also it is less pollutant and easily transportable. So from the report we say CNG is preferable as fuel.
![]()
Question 17.
Draw the diagram of candle flame and label all the zones. (OR)
Draw the diagram of structure of flame and label the parts. In which zone incomplete combustion takes place? (OR)
Draw a diagram of candle flame and label all the zones. What happens in the dark zone of a flame?
Answer:
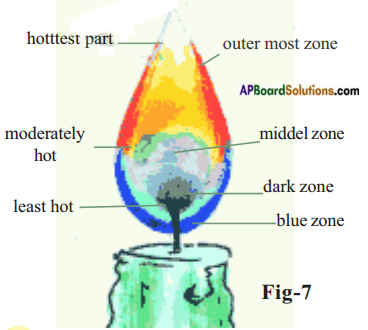
In the dark zone wax gets vapourised and it is least hot part, (or)
In black zone in complete combustion takes place.
Question 18.
Where do you find spontaneous combustion and rapid combustion in your daily life?
Answer:
Spontaneous combustion is observed in burning of match stick. When the match stick is struck against the rubbing surface the red phosphorous converts into white phosphorous which immediately react with potassium chlorate in the match stick head to produce enough heat to ignite antimony sulphide and start the combustion.
Rapid combustion is observed in gas stoves. When we turn the nob of the gas stove in the kitchen and bringing a burning match stick near to it. The gas burns rapidly and produce heat and light.
Question 19.
How do you organize your daily works with fuels to conserve bio-diversity?
Answer:
- I would use bicycle to travel short distances which consumes fuel.
- I would go to school or office (long distance) in a public vehicle like bus which will consume fuel.
- I will drive at a constant and moderate speed as far as possible which will consume fuel.
- I switch off the engine at traffic lights or at a place where I have to wait which will consume fuel.
- I ensure correct tyre pressure which will consume fuel.
- I ensure regular maintenance of the vehicle which decreases the harmful gases.
- I will clean gas burners regularly which decreases pollution.
- I will cook with sufficient water which will consume fuel.
- I would not prepare fries which take more time.
- I will use CNG in place of petrol or diesel which is less pollutant.
The above steps not only reduce the consumption of fuel and also decrease the air pollution. These steps of preserving fuels helps the mankind from pollution there by helps the bio-diversity.
![]()
Question 20.
How do you feel about “Fuels have become a part of human life”
Answer:
I feel that fuels are essential part of human life in their day to day life. Starting from the morning before they going to sleep they would found application of fuels. In the morning for breakfast we need fuel for cooking. For seeing TV or working with computer we need fuel because they run by electricity from thermal power (coal). If we want to go to school or office we use vehicle which required fuel. Every electrical articles like fans, mixies works by taking electricity from coal. So we conclude that fuels are essential part of human life.
Question 21.
It is difficult to bum a heap of green leaves but not a heap of dry leaves. Explain why?
Answer:
Green leaves contain water has high ignition temperature. So it will not burn whereas dry leaves does not contain water. So their ignition temperature is low. So they burn during summer.
Question 22.
In a few years the fuels on earth will be exhausted. Think, what would happen to human civilization? (OR)
In few years the fuel on earth will be exhausted. Predict the consequences.
Answer:
If people unable to use alternative sources of energy then lack of fuels drastically affect the human civilization. The consequences of lack of fuel.
- There would be no fuel for cooking.
- There would be no fuel for transport.
- There would be no fuel for running machinery.
- There would be no fuel for producing electricity.
So life of people on earth is miserable. The people once again go back to stone age where there is no availability of any facilities.
Question 23.
Use of more fuels in our daily life causes air pollution and it is harmful to human being and the other life on earth. Suggest some remedies to avoid this.
Answer:
Remedies to avoid air pollution:
- Look for alternative sources of energy like wind energy, solar energy, tidal energy which does not produce pollution.
- Try to minimise the use of fuels that is for travelling short distances use bicycles and try to go by bus (public transport) for offices which will minimise the use of fuels and there by air pollution.
- Ensure regular maintenance of vehicle and engine which will release the less quantity of harmful gases there by decrease of air pollution.
- Use CNG (compressed natural gas) as fuel as it is less pollutant and release small quantity of harmful gases.
![]()
Question 24.
Project work: Collect information about the experiments of Joseph Priestly. Write a two page report describing Priestly’s experiments proving that oxygen is needed for burning.
Answer:
In August 1774, Priestly isolated an air to be completely new, but he did not have an opportunity to pursue the matter because he was about to tour Europe. While in Paris Priestly managed to replicate the experiments for others, including French chemist Antonie Levoisier. After returning to Britain in January 1775, he continued his experiments and discovered vitriolic acid air (Sulphurdioxide, SO2).
In March he wrote to several people regarding the new air that he had discovered in August. One of these letters was read aloud to the royal society, and a paper outlining the discovery, titled “An Account of further Discoveries in air”, was published in society’s journal “Philosophical Transactions”. Priestly called the new substance “dephlogisticated air” which he made in the famous experiment by focussing sun’s rays on a sample of mercuric oxide. He first tested it on mice, who surprised him by surviving quite a while entrapped with the air, and then on himself, writing that it was “five or six better than common air for the purpose of respiration, inflammation and I believe, every other use of common atmospherical air. He had discovered oxygen gas (O2).”
Priestly called his discovery “dephlogisticated air” on the theory that it supported combustion so well because it had no phlogiston in it and hence could absorb maximum amount during burning.
Question 25.
Collect the information about annual fuel consumption in different parts of the world. How many years more the fossil fuels last? Make a poster with this information and issue an appeal to save fuel.
Answer:
Table showing different countries and their petrol consumption.
| Country | Usage of Petrol/Diesel per annum in barrels |
| United States | 6821850000 |
| China | 2993000000 |
| Japan | 1592495000 |
| India | 1087700000 |
| Russia | 1000100000 |
| Brazil | 897900000 |
| Germany | 889505000 |
| Saudi Arabia | 886950000 |
| South Korea | 797525000 |
The information showing the remaining years in which fossil fuels exhausted.
| Fuel | Years in which it exhausted |
| Petrol / Diesel | Around 60 years |
| Natural gas | 50 years |
| Coal | 250 years |
| LPG | Around 70 years |
We are using excess of petroleum products, coal and natural gas from the above table shows they will not exists long. So fuel consumption should be minimised otherwise no fuel is available in the future. So save fuel for better living.
8th Class Physical Science 8th Lesson Combustion, Fuels and Flame InText Questions and Answers
8th Class Physical Science Textbook Page No. 110
![]()
Question 1.
Why does candle give flame when it is burnt but why does coal burn without emitting a flame?
Answer:
A candle is made of wax in which a thick thread is inserted. When it is lighted melts by a match stick. A little of the wax forms gas. This gas combines with oxygen in the air to form flame.
But coal is a natural fuel contains more carbon. When it is burnt it sends out carbon monoxide and carbon dioxide gases and becomes ember and does not emit a flame. Coal is the changed material of wood. Hence it does not emit a flame as it possesses a huge calorific value (heat).
Question 2.
Do all fuels produce same amount of heat when they are burnt?
Answer:
No, different fuels produce different amount of heat when they are burnt.
Question 3.
What do we need to burn a material?
Answer:
We need oxygen to burn a material.
Question 4.
Have you ever tried burning a piece of paper or wood or coal, a small rock or a pebble?
Answer:
Yes, I tried.
Question 5.
Do all of them burn?
Answer:
Except a small rock and a pebble, the rest of them are burnt.
8th Class Physical Science Textbook Page No. 112
Question 6.
How does scented stick started burning?
Answer:
The stick starts burning with flame because oxygen supports combustion.
![]()
Question 7.
Why does not it catch again fire when it is kept aside in air after putting its flame off?
Answer:
The air is not sufficient to burn the scented stick.
8th Class Physical Science Textbook Page No. 113
Question 8.
a) A slow fire bursts into a flame when air is blown on it, but a candle burning with flame goes off when air is blown on it. Why?
Answer:
A slow fire burst into a flame when air is blown on it because it increase the supply of oxygen to fire. So combustion takes place to produce a flame.
Whereas when the wind or your breathe blows on the mixture of combustible particles and flame of a candle, it pushes the mixture of combustible particles away from the steam of fresh particles. So flame will be go out in the absence of combustible particles.
b) If a large quantity of dry grass is set on fire in forests then it is very difficult to put off the fire. Why?
Answer:
The dry grass is more combustible and supports burning. Hence due to the flow of oxygen (air) it is kindled.
Question 9.
When an object catches fire, the fire is put off by covering with sand or a blanket. Why ?
Answer:
Combustion takes place only in the presence of oxygen. When we cover a burning object with sand or blanket that would cut off the supply of oxygen to burning object. So the fire is puts off.
8th Class Physical Science Textbook Page No. 114
Question 10.
What makes match sticks to catch fire?
Answer:
A mixture of antimony trisulphide, potassium chlorate and white phosphorous with some glue and starch was applied on the head of a match stick made of suitable wood. When it struck against a rough surface white phosphorous got ignited due to heat of friction. This starts the combustion of match stick.
8th Class Physical Science Textbook Page No. 115
![]()
Question 11.
List different fuels that are used for!
Domestic purpose LPG, CNG
Automobiles/ Aircraft/ Trains/ Rockets Petrol, gasoline
Industry Coal, Charcoal, wood, natural gas
Classify the above fuels into solid, liquid, gases and write them in table.
| Solid | Liquid | Gas |
| Coal | Petrol | LPG |
| Charcoal | Gasoline | CNG |
| Wood | Diesel | Natural gas |
Look at the fuels in the above table.
Classify the above fuels into solid, liquid, gases and write them in table.
a) Can you decide the best fuel among them?
Answer:
CNG, LPG.
b) What is the criteria to decide a best fuel?
Answer:
CNG & LPG burn completely. The fuel which burns completely is the best fuel.
8th Class Physical Science Textbook Page No. 116
Question 12.
What is calorific value? Write its units.
Answer:
Calorific value of a fuel is the amount of heat energy produced on complete combustion of 1 kg of that fuel.
It is measured in kJ/kg.
Question 13.
How can we put off the fire if it breaks out?
Answer:
We can put off the fire by using the fire extinguishers in which CO2 gas is present.
Question 14.
How water helps in eliminating the factors, which support the combustion?
Answer:
The water spray cools the combustible material so that its temperature decreases. This prevents the fire from spreading.
![]()
Think and Discuss
8th Class Physical Science Textbook Page No. 111
Question 1.
Why some materials burn and why some do not? Give reasons.
Answer:
Burning is a process of reaction of a material with oxygen. The materials which have weak molecular bonds easily react with oxygen so they burn easily. The material which have strong molecular bonds does not react with oxygen easily because the molecular bonds should be broken before they react with oxygen.
Question 2.
Why some materials which do not burn at normal temperature burn at higher temperatures?
Answer:
Burning is a process of reaction of a material with oxygen. The material which have strong molecular bonds does not undergo burning because the molecular bonds should be broken in order to react the material with oxygen. So they have high ignition temperature. So the materials which do not burn at normal temperature burn at higher temperature.
8th Class Physical Science Textbook Page No. 112
Question 3.
If you lift the glass tumbler (which is placed over a burning candle) to 1 cm height what happens? Why?
Answer:
The Candle tends to burn because of availability air containing oxygen because cool air is more denser than hot air.
8th Class Physical Science Textbook Page No. 113
Question 4.
How do you say that the gas released in the above experiment is oxygen?
Answer:
If we put a burning match stick into the container having oxygen it burns with bright flame the reason is oxygen supports combustion.
Question 5.
Can we replace potassium permanganate with any other substance to release oxygen?
Answer:
We can replace with HgO (Mercuric oxide), KClO3 (Potassium chlorate), H2O2 (Hydrogen peroxide), NaNO3 (Sodium nitrate), KNO3 (Potassium nitrate) for release of oxygen.
Question 6.
Is there any other procedure to prove that oxygen is needed for burning?
Answer:
We can put off a fire by covering with sand or blanket because we are preventing supply of oxygen. So we can say oxygen is needed for burning.
8th Class Physical Science Textbook Page No. 115
![]()
Question 7.
Why is phosphorous preserved in water? (Hint: Think about the role of ignition temperature in combustion)
Answer:
Phosphorous is highly reactive with air containing oxygen. It has low ignition temperature. So it catches fire at room temperature. So it is preserved in water.
Question 8.
Why kerosene stoves and Bunsen burners have small holes in them? (Hint: Think about the role of air in combustion)
Answer:
Kerosene stoves and bunsen burners have small holes for entry of air for combustion of fuel.
Question 9.
It is hard to ignite match stick in rainy days. Why?
Answer:
It is hard to ignite match stick in rainy days because water has extinguishing property.
8th Class Physical Science Textbook Page No. 118
Question 10.
A wax candle burns with a yellow flame. The domestic gas burns with a blue flame. Why?
Answer:
The wax candle burns with a yellow in the middle zone because it undergoes partial combustion whereas domestic gas burns with a blue flame because it undergoes complete combustion.
8th Class Physical Science 8th Lesson Combustion, Fuels and Flame Activities
![]()
Activity – 1
Question 1.
Do all materials bum?
You will need a pair of tongs, some metal or clay dishes and a candle or a spirit lamp. Using tongs, pick up a small piece of paper and bring it near to the lighted candle and keep it on flame as shown in figure.

Record your observations in table.
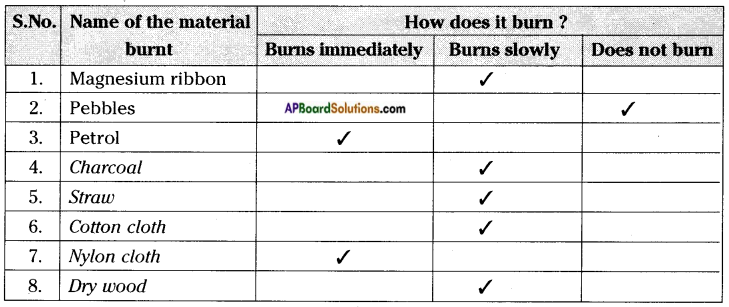
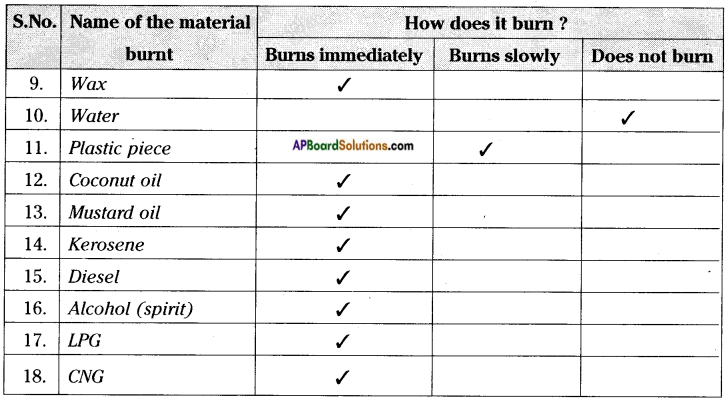
Carry out this experiment with a piece of charcoal, magnesium ribbon, straw, cotton cloth, nylon cloth, dry wood, pebble, wax, plastic piece, etc. and record your observations.
You can try to burn liquids like water, petrol, diesel, alcohol and note down your observations in table.
Take 2 ml of water in small plate. Bring the lighted stick near to water in plate.
a) What do you observe in your attempt of burning water?
Answer:
Water does not burn.
b) Is there any difference in flame of lighted stick?
Answer:
If we brought near to water plate the flame decreases.
![]()
c) What happened to the lighted stick when it is brought closer to water in the plate?
Answer:
The water in the plate puts off.the lighted stick.
Carryout this activity using coconut oil, mustard oil, kerosene, etc. Record your observation in the above table.
d) What can we conclude from this activity?
Answer:
We conclude that some materials burn and others do not.
e) Which of the material in the above activity are combustible?
Answer:
Except pebbles and water remaining materials are combustible.
Activity – 2
Question 2.
Testing the necessity of air for burning.
Answer:

Take a small burning candle and put it on a table. Invert a glass tumbler over it.
The candle continues to burn for some time. Then flickers and finally flame goes off.
Remove the tumbler and again light the candle. Put the tumbler back over the candle. When the candle flame begins to flicker, remove the tumbler. What happens to the candle?
We find that putting the glass tumbler over the candle cuts off the supply of air and candle flame goes off. When the flame just begins to flicker if we removes tumbler the flame retains back due to supply of oxygen.
This experiment proves that air is needed to burn a material.
Lab Activity
Write an activity to prove that oxygen helps in burning. (OR)
How can you perform the acitivity to prove that Oxygen is essential for burning? Explain.
Answer:
Aim: To prove that oxygen helps in burning.
Material required: Test tube, test tube holder, spirit lamp, match box, inscence stick (agarbatti), potassium permanganate (KMnO4) crystals.
Procedure:
Light a scented / incense stick and let it burn for 10 seconds, then put out the flame and keep it aside.
Take potassium permanganate (KMnO4) in a test tube. Hold the test tube with a test tube holder and heat it over the flame of spirit lamp. Oxygen is released on heating of potassium permanganate (KMnO4).
2 KMnO4 → K2MnO4 + MnO2 + O2 ↑
Insert the agarbatti with the burning stub into the test tube as shown in figure.
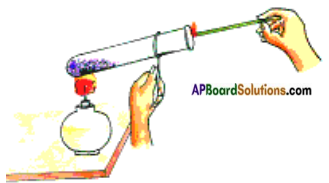
Observations: The stick burns with a flame. This proves that oxygen supports combustion by helping agarbatti to burn with bright flame.
Activity – 3
Question 3.
Burning a paper with sun rays.
On a sunny day, go out and focus the sun rays on a piece of paper using a magnifying lens.

a) Touch the spot after some time. How do you feel?
Answer:
It is hot.
You must have heard about people in ancient times rubbing the pieces of stones together to produce sparks.
b) Have you tried it?
Answer:
Yes.
![]()
c) Rub two stones together hardly and touch them. What do you feel?
Answer:
They become hot.
Now recall some of your experiences:
d) Does a matchstick burns by itself?
Answer:
No.
e) Why do you rub the match stick on the side of the matchbox to bum it?
Answer:
When we rub match stick on the side of the match box due to friction it produces it which will burn the match stick.
f) Can you bum a piece of wood by bringing it close to a lighted matchstick?
Answer:
No.
g) Why do we use paper pieces or kerosene oil to start fire in wood or coal?
Answer:
Paper or Kerosene oil has low ignition temperature. So they are used to burn wood or coal which have higher ignition temperature.
Activity – 4
Question 4.
Understanding ignition temperature.
Take two small paper cups. Pour water in one of the cups. Put the two cups on different tripod stands and heat both of them using a candle.

a) Which cup bums first?
Answer:
The cup which does not have water.
b) Does the water in the cup become hot? Why?
Answer:
The water in the second cup is hot because the heat received by second cup is transferred to water in it. The water in this cup prevents the paper to reach its ignition temperature and hence it does not burn.
c) When does the second cup start burning?
Answer:
When it reaches its ignition temperature.
![]()
Activity – 5
Question 5.
Observing the behaviour of different solid fuels.
Collect some fuels like candle, coal, charcoal, magnesium ribbon, wood, cakes of cow-dung, camphor, wick of the oil lamp, wick of kerosene stove, domestic gas, etc. Burn each of them one by one with the help of spirit lamp and note the time they take to catch fire. Also observe how do they burn?
a) Do all of them bum in the same manner? If not, what difference do you notice?
Answer:
No, some of them produce flame and some does not produce flame.
b) Do all of them form a flame while they are burning?
Answer:
No, all of them does not form flame.
Record your observation in the following table
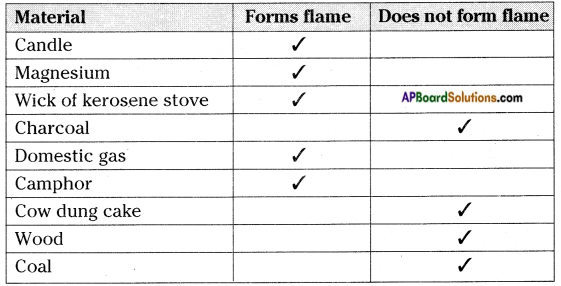
You may observe that a candle burns with flame whereas charcoal does not. Some materials burn with flame, some do not. Kerosene oil and molten wax rise through the wick become gas and form flames. But charcoal cannot be vaporized. So it does not produce flame.
Activity – 6
Question 6.
Observing the structure of the flame.
Light a wax candle and watch the flame.

Carefully note the different coloured zones in the flame.
a) How many colours are there in the flame?
Answer:
There are three colours in the flame.
b) Starting from the base of the flame, how many flame zones do you observe?
What is the colour of the outer most zone of the flame?
Answer:
There are three flame zones are observed.
The colour of outermost zone is blue.
c) Observe the innermost zone which is dark. What do you observe there?
Answer:
In this zone wax gets vapourised. This is dark zone.
Observe near the base of the flame. Vaporized wax gets completely oxidized and burns with a blue flame. It is blue zone.
![]()
Activity – 7
Question 7.
Observing what happens in different zones of candle flame.
Light a candle. Hold a glass tube (with 7 cm length) a pair of tongs and introduce its one end in the dark zone of a non flickering candle flame. Keep lighted match stick near the other end of the glass tube as shown in the figure.
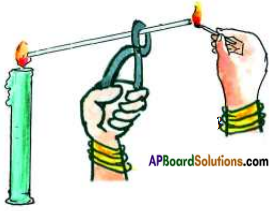
a) What do you observe? Do you see a flame? If so what is it, that produces a flame?
Answer:
Yes, the some of vapours produced by wax pass through the glass tube and are burnt by match stick to produce flame.
b) Notice that the wax near the heated wick melts quickly. What do you observe?
Answer:
When the candle’s flame is steady introduce a clean glass slide into the luminous zone of the flame and hold it with a pair of tongs for 10 seconds.
A blackish circular ring is formed on the glass slide.
c) What is it?
Answer:
The deposition of unburnt carbon particles present in the luminous zone of the flame due to incomplete combustion.
d) Hold a thin long copper wire just inside the flame for about half a minute. What do you observe? What is your inference?
Answer:
The copper wire just outside the flame gets red hot. It indicates that the non luminous zone of the flame has high temperature. It is the hottest part of the flame. It is blue in colour and complete combustion takes place due to good supply of oxygen.