AP State Board Syllabus AP SSC 10th Class Telugu Solutions 10th Class Telugu Grammar Sandhulu సంధులు Notes, Questions and Answers.
AP State Syllabus SSC 10th Class Telugu Grammar Sandhulu సంధులు
తెలుగు సంధులు
నా చిన్నప్పుడు చేసిన పనులు గుర్తుకు వచ్చాయి.
గమనిక :
పై వాక్యంలో “చిన్నప్పుడు” అనే పదం, చిన్న + అప్పుడు అనే రెండు పదాలు కలవడం వల్ల వచ్చింది. దీనినే “సంధి పదం” అంటారు. ఉచ్చరించడంలో సౌలభ్యం కోసం రెండు పదాలను వెంట వెంటనే కలిపి మాట్లాడవలసినప్పుడు, లేదా రాయవలసినప్పుడు “సంధి పదం” ఏర్పడుతుంది.
సంధి :
వ్యాకరణ పరిభాషలో రెండు స్వరాల (అచ్చుల) కలయికను “సంధి” అని పిలుస్తారు.
తెలుగు సంధులు :
రెండు తెలుగుపదాల మధ్య జరిగే సంధులను “తెలుగు సంధులు” అంటారు.
సంధి కార్యం : రెండు అచ్చుల మధ్య జరిగే మార్పును “సంధి కార్యం” అని పిలుస్తారు.
పూర్వ స్వరం :
సంధి జరిగే మొదటి పదం చివరి అక్షరంలోని అచ్చును (స్వరాన్ని) “పూర్వ స్వరం” అని పిలుస్తారు.
పర స్వరం :
సంధి జరిగే రెండవ పదం మొదటి అక్షరంలోని అచ్చును (స్వరాన్ని) “పర స్వరం” అని పిలుస్తారు.
ఉదా :
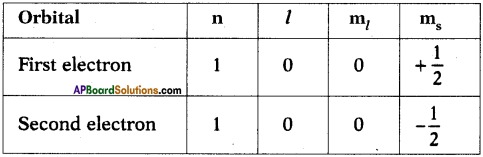
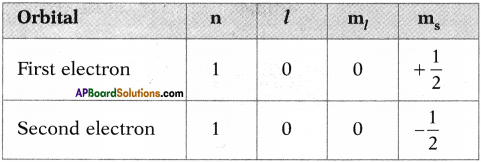
రామ + అయ్య : ‘రామ’ లోని ‘మ’ లో ‘అ’ పూర్వ స్వరం, ‘అయ్య’ లోని ‘అ’ పర స్వరం.

1. అత్వ సంధి సూత్రం
అత్తునకు సంధి బహుళంగా వస్తుంది.
ఈ కింది పదాలను విడదీయండి.
ఉదా : మేనల్లుడు = మేన + ‘అల్లుడు – (న్ +) అ + అ = అ) = (అత్వ సంధి)
1) ఒకప్పుడు : ఒక అప్పుడు = (అత్వ సంధి)
2) వచ్చినందుకు – వచ్చిన అందుకు : (అత్వ సంధి)
3) రాకుంటే ఉంటే (అత్వ సంధి)
4) లేకేమి = లేక + ఏమి = (అ + ఏ = (అత్వ సంధి)
5) పోవుటెట్లు : పోవుట + ఎట్లు = (అ + ఎ (అత్వ సంధి)
6) కొండంత = కొండ + అంత = (అ + ఎ (అత్వ సంధి)
గమనిక :
పై సంధి పదాలలోని పూర్వ స్వరం ‘అ’. అది పర స్వరంలోని అచ్చుతో కలిస్తే, పూర్వ స్వరం ‘అ’ లోపిస్తుంది. పై ఉదాహరణలలో ‘అ’ లోపించింది కాబట్టి ఇది ‘అత్వ సంధి’.
దీనిని అత్వ సంధి లేక ‘అకార సంధి’ అంటారు. పొట్టి ‘అ’ అనే అక్షరానికి, అచ్చు పరమైతే ‘అత్వ సంధి’ వస్తుంది.
2. ఇత్వ సంధి సూత్రం
ఏమ్యాదుల ఇత్తునకు సంధి వైకల్పికంగా వస్తుంది.
ఏమ్యాదులు = ఏమి మొదలగునవి.
ఏమి, మణి, కి (షష్ఠి, అది, అవి, ఇది, ఇవి, ఏది, ఏవి మొదలైనవి ఏమ్యాదులు. (కి షష్ఠి అంటే ‘కిన్’)
ఈ కింది పదాలను విడదీయండి.
ఉదా :
అ) ఏమంటివి = ఏమి + అంటివి = (ఇ + అ = అ) = (ఇత్వ సంధి)
సంధి జరుగనప్పుడు “య” కారం ఆగమంగా వస్తుంది. దానినే ‘యడాగమం’ అని పిలుస్తారు.
ఆ) ఏమియంటివి = ఏమి + య్ + అంటివి = ఇ + (య్ + అ) = య (ఇకార సంధి రాని యడాగమ రూపం)
గమనిక :
ప్రథమ, ఉత్తమ పురుష బహువచన క్రియల ఇకారానికి, సంధి వైకల్పికంగా జరుగుతుంది.
వచ్చిరిపుడు = వచ్చిరి + ఇపుడు – (ఇ + ఇ + ఇ) = (ఇత్వ సంధి)
వచ్చిరియిపుడు = వచ్చిరి + య్ + ఇపుడు – (ఇ + ఇ + యి) (యడాగమం వచ్చిన రూపం)
గమనిక :
పై ఉదాహరణలలో హ్రస్వ ‘ఇ’ కారానికి అచ్చు కలిసినపుడు సంధి జరిగింది. దీనిని “ఇత్వ సంధి” అంటారు. ఇత్వ సంధి తప్పక జరగాలన్న నియమం లేదు.
వైకల్పికం :
సంధి జరుగవచ్చు లేక జరుగకపోవచ్చు. వ్యాకరణంలో ఈ పరిస్థితిని “వైకల్పికం” అని పిలుస్తారు.
అభ్యాసం :
ఉదా : 1) ఏమంటివి = ఏమి + అంటివి : (మ్ + ఇ + అ = మ) : ఇత్వ సంధి
2) పైకెత్తినారు : పైకి + ఎత్తినారు : (ఇ + ఎ = ఎ) : ఇత్వ సంధి
3) మనిషన్నవాడు = మనిషి + అన్నవాడు – (ఇ + అ = అ) – ఇత్వ సంధి
3. ఉత్వ సంధి సూత్రం
ఉత్తునకు అచ్చు పరమైనపుడు సంధి నిత్యంగా వస్తుంది.
ఈ కింది పదాలను విడదీయండి.
ఉదా : రాముడతడు = రాముడు + అతడు = (డ్ + ఉ + అ = డ) : (ఉత్వ సంధి)
1) అతడెక్కడ = అతడు + ఎక్కడ = (A + ఎ = ఎ) : (ఉత్వ సంధి)
2) మనమున్నాము = మనము + ఉన్నాము : (ఉ + ఉ = ఉ) : (ఉత్వ సంధి)
3) మనసైన . మనసు + ఐన = (A + ఐ = ఐ) : . (ఉత్వ సంధి)
గమనిక :
హ్రస్వ ఉ కారానికి, అనగా ఉత్తునకు, అచ్చు కలిసినప్పుడు, పూర్వ స్వరం ‘ఉ’ కారం లోపించి, పర స్వరం కనిపిస్తుంది. లోపించిన పూర్వ స్వరం ‘ఉ’ కాబట్టి, ఇది “ఉత్వ సంధి” అని పిలువబడుతుంది.
నిత్యం :
నిత్యం అంటే, తప్పక సంధికార్యం జరుగుతుందని అర్థం.
4. యడాగమ సంధి సూత్రం
సంధి లేనిచోట అచ్చుల మధ్య “య్” వచ్చి చేరడాన్ని “యడాగమం” అని పిలుస్తారు.
ఈ కింది పదాలను విడదీయండి.
ఉదా :
అ) మాయమ్మ = మా + అమ్మ – మాయమ్మ
ఆ) మాయిల్లు = మా + ఇల్లు = మాయిల్లు
ఇ) హరియతడు = హరి + అతడు = హరియతడు
గమనిక :
పై ఉదాహరణలలో సంధి జరుగలేదు. కాని కొత్తగా ‘య్’ వచ్చి చేరింది. అలా చేరడం వల్ల ఈ కింది విధంగా మార్పు జరిగింది.
అ) మా + య్ + అమ్మ : మా ‘య’ మ్మ
ఆ) మా + య్ . + ఇల్లు : మా ‘ఋ’ ల్లు
ఇ) హరి + య్ + అతడు = హరి ‘య’ తడు
5. ఆమ్రేడిత సంధి సూత్రం
అచ్చునకు ఆమ్రేడితం పరమైతే సంధి తరచుగా వస్తుంది.
ఆమ్రేడితం :
ఒక పదాన్ని రెండుసార్లు ఉచ్చరిస్తే, రెండోసారి ఉచ్చరింపబడిన పదాన్ని ‘ఆమ్రేడితం’ అంటారు.
ఉదా :
1) ఆహాహా = ఆహా + ఆహా – ‘ఆహా’ అనే పదం రెండుసార్లు వచ్చింది. అందులో రెండవ ‘ఆహా’ అనే దాన్ని ఆమ్రేడితం అని పిలవాలి.
2) అరెరె : అరె అరె : రెండవసారి వచ్చిన ‘అరె’ ఆమ్రేడితం.
3) ఔరౌర – ఔర + . ఔర = రెండవసారి వచ్చిన ‘ఔర’ ఆమ్రేడితం.
గమనిక :
పై ఉదాహరణలలో ఒక్కొక్క పదం రెండుసార్లు వచ్చింది. రెండవసారి వచ్చిన పదాన్ని ‘ఆమ్రేడితం’ అంటారు.
ఆమ్రేడిత సంధికి ఉదాహరణములు :
ఔర + ఔర = (ఔర్ + అ)
ఆహా + ఆహా = (ఆహ్ + ఆ)
ఓహో + ఓహో = (ఓహ్ + ఓ)
గమనిక :
పై ఉదాహరణలలో పూర్వ పదం అనగా మొదటి పదం చివర, అ, ఆ, ఓ వంటి అచ్చులున్నాయి. ఈ అచ్చులకు ఆమ్రేడితం పరమైతే, సంధి వస్తుంది.
ఔర + ఔర = ఔరౌర = (అ + ఔ = ఔ)
ఆహా + ఆహా = ఆహాహా = (ఆ + ఆ = ఆ)
ఓహో + ఓహో = ఓహోహో = (ఓ + ఓ = ఓ)
ఏమి + ఏమి = ఏమేమి = (ఇ + ఏ = ఏ)
ఎట్లు + ఎట్లు = ఎట్లెట్లు = (ఉ + ఎ = ఎ)
ఏమిటి + ఏమిటి = ఏమిటేమిటి = (ఇ + ఏ = ఏ)
అరె + అరె = అరెరె = (ఎ + అ = ఎ)
గమనిక :
ఆమ్రేడిత సంధి, ఈ కింది ఉదాహరణలలో వికల్పంగా జరుగుతుంది. వీటిని గమనిస్తే, సంధి జరిగిన రూపం ఒకటి, సంధిరాని రూపము మరొకటి కనబడతాయి.
ఉదా :
ఏమి + ఏమి = ఏమేమి, ఏమియేమి (సంధి వైకల్పికం)
ఎట్లు + ఎట్లు – ఎబ్లెట్లు, ఎట్లుయెట్లు (సంధి వైకల్పికం)
ఎంత + ఎంత = ఎంతెంత, ఎంతయెంత (సంధి వైకల్పికం)

6. ఆమ్రేడిత ద్విరుక్తటకారాదేశ సంధి సూత్రం
ఆమ్రేడితం పరమైనపుడు, కడాదుల తొలి యచ్చు మీది వర్ణాల కెల్ల అదంతమైన ద్విరుక్తటకారం వస్తుంది.
కడాదులు (కడ + ఆదులు) = కడ, ఎదురు, కొన, చివర, తుద, తెన్ను, తెరువు, నడుము, పగలు, పిడుగు, బయలు, మొదలు, మొదలైనవి కడాదులు.
కింది ఉదాహరణలను గమనించండి.
1) పగలు + పగలు = పట్టపగలు
2) చివర + చివర = చిట్టచివర
3) కడ + కడ = కట్టకడ
గమనిక :
1) పగలు + పగలు – పట్టపగలు అవుతోంది. అంటే ‘ప’ తర్వాత ఉన్న ‘గలు’ అన్న అక్షరాలకు బదులుగా, ‘ఋ’ వచ్చింది. ‘మీ’ వచ్చి, ‘పట్టపగలు’ అయింది.
2) చివర + చివర అన్నప్పుడు ‘చి’ తర్వాత రెండక్షరాల మీద ‘జ’ వచ్చి, ‘చిట్టచివర’ అయింది.
3) కడ + కడ అన్నప్పుడు ‘డ’ స్థానంలో ‘జ’ వచ్చి ‘కట్టకడ’ అయింది. ఇప్పుడు కిందివాటిని కలిపి రాయండి.
ఎదురు + ఎదురు = ఎట్టయెదురు
కొన + కొన = కొట్టకొన
మొదట + మొదట = మొట్టమొదట
బయలు + బయలు = బట్టబయలు
తుద + తుద : తుట్టతుద
గమనిక :
ఆమ్రేడితం పరంగా ఉంటే, కడ మొదలైన శబ్దాల మొదటి అచ్చు మీద ఉన్న అన్ని అక్షరాలకు, ‘ఋ’ (ద్విరుక్తటకారం) వస్తుండడం గమనించాం.
7. ద్రుతప్రకృతిక సంధి సరళాదేశ సంధి
ద్రుతప్రకృతికం మీది పరుషాలకు సరళాలు వస్తాయి.
ఈ కింది పదాలు చదివి, పదంలోని చివర అక్షరం కింద గీత గీయండి. 1) పూచెను 2) చూచెన్ 3) తినెను 4) చేసెన్ 5) ఉండెన్
గమనిక :
పై పదాలను గమనిస్తే పదాల చివర, ను, న్ లు కనిపిస్తాయి. అంటే పదాల చివర నకారం ఉంది. ఈ నకారాన్ని ‘ద్రుతం’ అంటారు. ద్రుతము చివర గల పదాలను, “ద్రుతప్రకృతికాలు” అంటారు.
గమనిక :
పూచెను, చూచెన్, తినెను, చేసెన్, ఉండెన్ – అనేవి ద్రుతప్రకృతికాలు
కింది ఉదాహరణములను గమనించండి.
ఉదా:
అ) పూచెన్ + కలువలు = పూచెన్ + గలువలు
ఆ) దెసన్ + చూచి = దెసన్ + జూచి
ఇ) చేసెన్ + టక్కు = చేసెన్ + డక్కు
ఈ) పాటిన్ + తప్ప : పాటిన్ + దప్ప
ఉ) వడిన్ + పట్టి = వడిన్ + బట్టి
ఊ) చేసెను + తల్లీ : ‘ ‘ చేసెను + దల్లీ
ఋ) దెసను + చూసి : దెసను + జూసి
గమనిక :
ద్రుతప్రకృతికానికి ‘క’ పరమైతే ‘గ’, ‘చ’ పరమైతే ‘జ’, ‘ట’ పరమైతే ‘డ’, ‘త’ పరమైతే ‘ద’, ‘ప’ పరమైతే ‘బ’ ఆదేశంగా వస్తాయి.
1) క – ‘గ’ గా
2) చ – ‘జ’ గా
3) ట – ‘డ’ గా
4) త – ‘ద’ గా
5) ప – ‘బి’ గా మార్పు వచ్చింది.
ఇందులో ‘క చ ట త ప’ లకు, ‘పరుషాలు’ అని పేరు, ‘గ జ డ ద బ’ లకు, ‘సరళాలు’ అని పేరు.
దీనిని బట్టి సరళాదేశ సంధి సూత్రం ఇలా ఉంటుంది.
ద్రుత ప్రకృతిక సంధి సూత్రం (1):
ద్రుత ప్రకృతికం మీది పరుషాలకు, సరళాలు వస్తాయి.
గమనిక :
ఇప్పుడు పై ఉదాహరణలలో మార్పు గమనించండి.
ఉదా :
పూచెఁ గలువలు (ద్రుతం అరసున్నగా మారింది)
1) పూచెను + కలువలు (పూచెం గలువలు (ద్రుతం సున్నగా మారింది)
2) పూచెనలువలు (ద్రుతం మీద హల్లుతో కలిసి సంశ్లేష రూపం అయ్యింది)
3) పూచెను గలువలు. (ద్రుతం మార్పు చెందలేదు) దీనికి సూత్రం చెపితే సూత్రం ఇలా ఉంటుంది.
ద్రుత ప్రకృతిక సంధి సూత్రం (2) : ఆదేశ సరళాలకు ముందున్న ద్రుతానికి, బిందు, సంశ్లేషలు విభాషగా వస్తాయి.
గమనిక :
అంటే ఒక్కోసారి బిందువు వస్తుంది. ఒక్కోసారి సంశ్లేష వస్తుంది.
8. గసడదవాదేశ సంధి సూత్రం
ప్రథమ మీది పరుషాలకు గ,స,డ,ద,వ లు బహుళంబుగా వస్తాయి.
కింది పదాలను ఎలా విడదీశారో గమనించండి.
1) గొప్పవాడు = గదా – గొప్పవాడు + కదా (డు + క)
2) కొలువుసేసి = కొలువు + చేసి (వు + చే)
3) వాడు డక్కరి = వాడు + టక్కరి (డు + ట)
4) నిజము దెలిసి = నిజము + తెలిసి (ము + తె)
5) పాలువోయక = పాలు + పోయక (లు + పో)
గమనిక : పై ఉదాహరణలలో పూర్వపదం చివర, ప్రథమా విభక్తి ప్రత్యయాలు ఉన్నాయి. పరపదం మొదట క, చ, ట, త, ప, లు ఉన్నాయి. ఈ విధంగా ప్రథమావిభక్తి మీద ప్రత్యయాలు, క, చ, ట, త, ప లు పరమైతే, వాటి స్థానంలో గ, స, డ, ద, వ లు ఆదేశంగా వస్తాయి. అంటే
1) క – గ గా మారుతుంది.
2) చ – స గా మారుతుంది.
3) ట – డ గా మారుతుంది.
4) త – ద గా మారుతుంది.
5) ప – వ గా మారుతుంది.
అంటే క, చ, ట, త, ప లకు గ, స, డ, ద, వ లు ఆదేశంగా వస్తాయి.
ద్వంద్వ సమాసంలో : గసడదవాదేశ సంధి
కింది పదాలను గమనించండి.
కూరగాయలు = కూర + కాయ + లు
కాలుసేతులు = కాలు + చేయి + లు
టక్కుడెక్కులు = టక్కు + టెక్కు + లు
తల్లిదండ్రులు = తల్లి + తండ్రి + లు
ఊరువల్లెలు : ఊరు + పల్లె + లు
గమనిక :
పై ఉదాహరణలు ద్వంద్వ సమాసపదాలు. పై ఉదాహరణలలో కూడా క చట త ప లకు, గసడదవ లు వచ్చాయి. దీన్నే గసడదవా దేశం అంటారు.
గసడదవాదేశ సంధి సూత్రం:
ద్వంద్వ సమాసంలో మొదటి పదం మీద ఉన్న క చట త ప లకు గసడదవలు క్రమంగా వస్తాయి.
కింది పదాలను కలపండి.
1) అక్క , చెల్లి : అక్కసెల్లెండ్రు
2) అన్న తమ్ముడు – అన్నదమ్ములు
9. టుగాగమ సంధి సూత్రం
కర్మధారయములందు ఉత్తునకు, అచ్చుపరమైతే టుగాగమం వస్తుంది.
ఈ కింది పదాలను పరిశీలించండి.
నిలువు + అద్దం = నిలువుటద్దం
తేనె + ఈగ = తేనెటీగ
పల్లె + ఊరు = పల్లెటూరు
గమనిక :
వీటిలో సంధి జరిగినపుడు ‘ట్’ అదనంగా చేరింది. ఇలా ‘ట్’ వర్ణం అదనంగా వచ్చే సంధిని, ‘టుగాగమ సంధి’ అంటారు. అలాగే కింది పదాలు కూడా గమనించండి. తేనె, పల్లె అనే పదాల చివర ‘ఉ’ లేక పోయినా, టుగాగమం వచ్చింది.
1) చిగురు + ఆకు = చిగురుటాకు / చిగురాకు
2) పొదరు + ఇల్లు : పొదరుటిల్లు / పొదరిల్లు
గమనిక :
వీటిలో ‘ట్’ అనే వర్ణం సంధి జరిగినపుడు రావచ్చు. ‘ట్’ వస్తే “టుగాగమం” అవుతుంది. ‘ట్’ రాకుంటే ‘ఉత్వ సంధి’ అవుతుంది.
సూత్రం :
కర్మధారయమునందు పేర్వాది శబ్దాలకు అచ్చు పరమైనపుడు, టుగాగమం విభాషగా వస్తుంది.
ఉదా :
1) పేరు + ఉరము = పేరు టురము / పేరురము
2) చిగురు + ఆకు = చిగురుటాకు / చిగురాకు
3) పొదరు + ఇల్లు = పొదరుటిల్లు / పొదరిల్లు

10. లులన సంధి సూత్రం
లు, ల, న లు పరమైనపుడు ఒక్కొక్కప్పుడు ‘ము’ గాగమానికి లోపమూ, దాని పూర్వ స్వరానికి దీర్ఘమూ విభాషగా వస్తాయి.
ఈ కింది ఉదాహరణములు గమనించండి.
1) పుస్తకములు – పుస్తకాలు
2) దేశముల – దేశాల
3) జీవితమున – జీవితాన
4) గ్రంథములు – గ్రంథాలు
5) రాష్ట్రముల – రాష్ట్రాల
6) వక్షమున – వృక్షాన
పై పదాల్లో మార్పును గమనించండి.
పుస్తకములు, గ్రంథములు, దేశములు, రాష్ట్రములు, జీవితమున, వృక్షమున – వీటినే మనం పుస్తకాలు, గ్రంథాలు, దేశాలు, రాష్ట్రాలు, జీవితాన, వృక్షాన అని కూడా అంటాం.
గమనిక :
ఈ మార్పులో, లు,ల, న అనే అక్షరాల ముందున్న ‘ము’ పోయింది. ‘ము’ కంటే ముందున్న అక్షరానికి దీర్ఘం వచ్చింది.
11. పడ్వాది సూత్రం
పడ్వాదులు పరమైనపుడు ‘ము’ వర్ణకానికి లోప పూర్ణబిందువులు విభాషగా వస్తాయి.
ఈ కింది ఉదాహరణలు గమనించండి.
1) భయము + పడు = భయంపడు, భయపడు
విడదీసిన పదాలకూ, కలిపిన పదాలకూ తేడా గమనించండి. కలిపిన పదంలో ‘ము’ కు బదులుగా సున్న(0) వచ్చింది. మరో దానిలో ‘ము’ లోపించింది.
గమనిక :
పడ్వాదులు = పడు, పట్టె, పాటు అనేవి.
12. త్రిక సంధి సూత్రం
1. ఆ, ఈ, ఏ అనే సర్వనామాలు త్రికం అనబడతాయి.
2. త్రికం మీది అసంయుక్త హల్లుకు ద్వితం బహుళంగా వస్తుంది.
3. ద్విరుక్తమైన హల్లు పరమైనపుడు, ఆచ్చికమైన దీర్ఘానికి హ్రస్వం వస్తుంది.
ఈ కింది ఉదాహరణ చూడండి.
అక్కొమరుండు = ఆ + కొమరుండు
ఆ + కొమరుండు = అనే దానిలో, ‘ఆ’, త్రికంలో ఒకటి. ఇది ‘అ’ గా మారింది. సంయుక్తాక్షరం కాని హల్లు ‘కొ’ ద్విత్వంగా ‘క్కొ’ గా మారింది.
అలాగే ఈ, ఏ లు అనే త్రికములు కూడా, ఇ, ఎ లుగా మారతాయి.
ఉదా :
ఈ + కాలము = ఇక్కాలము
ఏ + వాడు = ఎవ్వాడు
త్రిక సంధి సూత్రం (1):
త్రికము మీది అసంయుక్త హల్లుకు ద్విత్వం బహుళంగా వస్తుంది.
ఉదా :
ఈ + క్కాలము
ఏ + వ్వాడు
త్రిక సంధి సూత్రం (2) : ద్విరుక్తమైన హల్లు పరమైనపుడు అచ్ఛిక దీరానికి హ్రస్వం అవుతుంది.
ఉదా : 1) ఇక్కాలము 2) ఎవ్వాడు

13. రుగాగమ సంధి
సూత్రం : పేదాది శబ్దాలకు ‘ఆలు’ శబ్దం పరమైతే, కర్మధారయంలో రుగాగమం వస్తుంది.
పేదాదులు = (పేద + ఆదులు) పేద మొదలైనవి.
పేద, బీద, ముద్ద, బాలెంత, కొమ, జవ, అయిదువ, మనుమ, గొడ్డు మొదలైనవి పేదాదులు.
ఉదా : పేద + ఆలు = పేద + ర్ + ఆలు = పేదరాలు
పై రెండు పదాలకు మధ్య ‘5’ అనేది వచ్చి, ప్రక్కనున్న ‘ఆ’ అనే అచ్చుతో కలిస్తే ‘రా’ అయింది. అదెలా వస్తుందంటే, పేద, బీద, బాలెంత ఇలాంటి పదాలకు ‘ఆలు’ అనే శబ్దం పరమైతే, ఇలా ‘రుగాగమం’ అంటే ‘5’ వస్తుంది. ఆగమం : రెండు పదాలలో ఏ అక్షరాన్ని కొట్టివేయకుండా, కొత్తగా అక్షరం వస్తే “ఆగమం” అంటారు.
మనుమ + ఆలు = మనుమరాలు
బాలెంత + ఆలు = బాలెంతరాలు
రుగాగమ సంధి సూత్రం (2) :
కర్మధారయంలో తత్సమ పదాలకు, ఆలు శబ్దం పరమైతే, పూర్వ పదం చివరనున్న అత్వానికి ఉత్వమూ, రుగాగమమూ వస్తాయి.
ఉదా :
ధీరురాలు = ధీర + ఆలు
గుణవంతురాలు = గుణవంత + ఆలు
విద్యావంతురాలు = విద్యావంత + ఆలు
14. పుంప్వాదేశ సంధి సూత్రం
కర్మధారయ సమాసాల్లో “ము” వర్ణకానికి బదులు “పుంపులు” ఆదేశంగా వస్తాయి.
గమనిక :
“ము” అనే వర్ణకానికి బదులుగా “పు” కాని, “ఎపు” కాని వస్తుంది. దీన్ని వ్యాకరణ దృష్టిలో “ఆదేశం” అని పిలుస్తారు. కింది పదాలు విడదీసి చూడండి. మార్పును గమనించండి.
ఉదా :
అచ్చపు పూలతోట = అచ్చము + పూలతోట
అ) నీలపుఁగండ్లు = నీలము + కండ్లు
ఆ) ముత్తెపుసరులు = ముత్తెము + సరులు
ఇ) సరసపుమాట = సరసము + మాట
గమనిక :
పైన పేర్కొన్న ఉదాహరణలలో రెండు మార్పులను మనం గమనించవచ్చు.
అ) మొదటి పదాల్లో ‘ము’ వర్ణకం లోపించింది.
ఆ) ప్రతి సంధి పదంలోనూ మొదటి పదం విశేషణాన్ని తెలుపుతుంది.
గమనిక :
సమాసంలో మొదటి పదం విశేషణం, రెండవ పదం విశేష్యం అయితే, ఆ సమాసాలను “విశేషణ పూర్వపద కర్మధారయ సమాసం” అంటారని మనకు తెలుసు. అంటే ఈ పుంప్వాదేశ సంధి, కర్మధారయ సమాసాల్లో ఏర్పడుతుందని గ్రహించాలి.
అభ్యాసం :
కింది పదాలను విడదీసి, సంధి సూత్రాన్ని సరిచూడండి.
అ) సింగపు కొదమ
జవాబు:
సింగపుకొదమ : సింగము + ‘కొదమ = పుంప్వాదేశ సంధి
గమనిక :
ఇక్కడ సింగము అనే మొదటి పదం చివర ఉన్న “ము” వర్ణకం పోయి, “పు” ఆదేశంగా వచ్చింది. కాబట్టి ఇది “పుంప్వాదేశ సంధి.”
ఆ) ముత్యపుచిప్ప
జవాబు:
ముత్యపుచిప్ప = ముత్యము + చిప్ప + పుంప్వాదేశ సంధి
గమనిక :
ఇక్కడ ముత్యము అనే మొదటి పదం చివర ఉన్న “ము” వర్ణకం పోయి, “పు” ఆదేశంగా వచ్చింది. కాబట్టి ఇది “పుంప్వాదేశ సంధి.”
ఇ) కొంచెపు నరుడు
జవాబు:
కొంచెపునరుడు = కొంచెము + నరుడు = పుంప్వాదేశ సంధి
గమనిక :
ఇక్కడ కొంచెము అనే పూర్వపదం చివర ఉన్న “ము” వర్ణకం పోయి, “పు” ఆదేశంగా వచ్చింది. కాబట్టి ఇది పుంప్వాదేశ సంధి.
15. ప్రాతాది సంధి సూత్రం
సమాసాలలో ప్రాతాదుల తొలి అచ్చు మీది వర్ణాలకెల్ల లోపం బహుళంగా వస్తుంది.
కింద గీత గీసిన పదాలను విడదీయండి. మార్పులు గమనించండి.
అ. పూరెమ్మ అందంగా ఉన్నది.
జవాబు:
పూరెమ్మ : పూవు + రెమ్మ – ప్రాతాది సంధి
ఆ. గురుశిష్యులు పూదోటలో విహరిస్తున్నారు.జవాబు:
పూదోట : పూవు + తోట = ప్రాతాది సంధి
ఇ) కొలనులో కెందామరలు కొత్త శోభను వెదజల్లుతున్నాయి.
జవాబు:
కెందామరలు – కెంపు + తామరలు = ప్రాతాది సంధి
ఈ) ఆ ముసలివానిలాగే అతని ప్రాయిల్లు జీర్ణమైయున్నది.
జవాబు:
ప్రాయిల్లు : పాత + ఇల్లు = ప్రాతాది సంధి
పై సంధి పదాల విభజనను సరిచూడండి. వచ్చిన మార్పు గమనించండి.
అ) పూవు + రెమ్మ = పూరెమ్మ = ప్రాతాది సంధి
ఆ) పూవు + తోట = పూఁదోట = ప్రాతాది సంధి
ఇ) కెంపు + తామరలు = కెందామరలు = ప్రాతాది సంధి
ఈ) ప్రాత + ఇల్లు = ప్రాయిల్లు = ప్రాతాది సంధి
ఉ) మీదు + కడ = మీఁగడ = ప్రాతాది సంధి
గమనిక :
1) ఈ ఐదు సందర్భాలలోనూ మొదటి పదంలోని మొదటి అక్షరం తరువాత ఉన్న వర్ణాలన్నీ లోపిస్తాయి.
2) రెండవ పదం మొదట ఉన్న పరుషా (త, క) లు, సరళా (ద, గ) లుగా మారాయి.
పై మార్పులను బట్టి, ప్రాతాది సంధి నియమాలను ఈ విధంగా అర్థం చేసుకోవచ్చు.
ప్రాతాది సంధి
ప్రాతాది సంధి సూత్రము (1):
సమాసాలలో ప్రాతాదుల తొలి అచ్చు మీది వర్ణాలకెల్ల లోపం బహుళంగా వస్తుంది.
1) పూవు + తోట = పూ + తోట
2) కెంపు + తామర = కెల + తామర
3) మీదు + కడ = మీ + కడ
ప్రాతాది సంధి సూత్రము (2) :
లోపింపగా మిగిలిన (సంధిలోని) మొదటి అక్షరానికి పరుషాలు పరమైతే నుగాగమం (‘న్’ ఆగమంగా) వస్తుంది.
1) పూ + న్ + తోట = పూదోట
2) కె +న్ + తామర = కెందామర
3) మీ + 5 + కడ = మీగడ
గమనిక :
నుగాగమంలోని ‘స్’ అనేది, పూర్ణబిందువుగా గాని, అర్థబిందువుగా గాని, సరళాదేశ సంధి వల్ల మారుతుంది.
ఉదా : 1) పూఁదోట
2) కెందామర
3) మీఁగడ
గమనిక :
ప్రాతాదులు అంటే ‘పాత’ మొదలయిన కొన్ని మాటలు. అవి
1) ప్రాత
2) లేత
3) క్రొత్త
4) క్రిందు
5) కెంపు
6) చెన్ను మొ||నవి.

16. నుగాగమ సంధి సూత్రం
ఉదంతమగు తద్ధర్మార్థక విశేషణానికి అచ్చు పరమైనపుడు నుగాగమం వస్తుంది.
అభ్యాసం :
కింది పదాలను విడదీయండి. మార్పును గమనించండి.
అ) నుగాగమ సంధి:
అ) చేయునతడు = చేయు + అతడు
ఆ) వచ్చునపుడు = వచ్చు + అపుడు
గమనిక :
చేయు, వచ్చు వంటి క్రియలు చివర ‘ఉత్తు’ను, అనగా హ్రస్వ ఉకారాన్ని కల్గి ఉంటాయి. కాబట్టి వీటిని ‘ఉదంతాలు’ అంటారు. ఇవి తద్ధర్మార్థక క్రియలు. అంటే ఏ కాలానికైనా వర్తించే క్రియలు. ఈ ఉదంత, తద్ధర్మార్థక క్రియలకు అచ్చు కలిసింది. అప్పుడు ‘న్’ అనే అక్షరం కొత్తగా వస్తుంది. ‘న్’ ఆగమంగా అనగా ఉన్న అక్షరాలను కొట్టి వేయకుండా వస్తుంది. కాబట్టి ఇది “నుగాగమ సంధి’.
ఉదా :
చేయు + అతడు = చేయు +న్ + అతడు = చేయునతడు
వచ్చు + అప్పుడు = వచ్చు + న్ + అప్పుడు = వచ్చునప్పుడు
గమనిక :
అతడు, అప్పుడు అనే పదాలలోని ‘అ’ అనే అచ్చు పరంకాగా, కొత్తగా ‘న్’ ఆగమంగా వచ్చి, నుగాగమం అయింది.
ఉదా :
1) వచ్చునప్పుడు
2) చేయునతడు
ఆ. నుగాగమ సంధి:
గమనిక :
ఈ పై సందర్భాలలోనే కాక, మరికొన్ని చోట్ల కూడా నుగాగమం వస్తుంది. కింది పదాలు విడదీసి, పరిశీలించండి.
అ) తళుకుం గజ్జెలు
ఆ) సింగపుం గొదమ
పై సంధి పదాలను విడదీస్తే
అ) తళుకుం గజ్జెలు – తళుకు + గజ్జెలు
ఆ) సింగపుం గొదమ = సింగము + కొదమ
గమనిక :
‘తళుకు గజ్జెలు’ అనే సంధి పదంలో ‘తళుకు’ అనే పదం, ఉత్తు చివర గల స్త్రీ సమశబ్దం. ఇటువంటి ఉదంత స్త్రీ సమపదాలకు, పరుషాలుగాని, సరళాలుగాని పరమైతే నుగాగమం వస్తుంది.
ఉదా :
తళుకు + 5 + గజ్జెలు = ద్రుతానికి సరళ స్థిరాలు పరమైతే పూర్ణబిందువు వస్తుంది. తళుకుం గజ్జెలు అవుతుంది.
అలాగే పుంపులకు, పరుష సరళాలు పరమైతే, నుగాగమం వస్తుంది.
గమనిక :
సింగపు + కొదమ అనే చోట ‘సింగపు’ అన్న చోట చివర ‘పు’ ఉంది. దానికి ‘కొదమ’ లో మొదటి అక్షరం ‘అ’ అనేది పరుషం పరమైంది. పుంపులకు పరుషం పరమైంది. కాబట్టి ‘నుగాగమం’ అనగా ‘5’ వస్తుంది.
ఉదా :
సింగపు + 5 + కొదమ సరళాదేశం రాగా సింగపుఁగొదమ అవుతుంది.
అభ్యాసం :
కింది ఉదాహరణలు పరిశీలించి, లక్షణ సమన్వయం చేయండి.
ఉదా :
తళుకుంగయిదువు – తళుకు + 5 + కయిదువు = సరళాదేశ సంధి రాగా, తళుకుం గయిదువు అవుతుంది.
గమనిక :
తళుకు అన్నది (ఉదంత స్త్రీ సమం); ‘కయిదువు’ పదంలో మొదట ‘క’ అనే పరుషము ఉంది. కాబట్టి నుగాగమం వస్తుంది.
1. చిగురుం గయిదువు
జవాబు:
చిగురుం గయిదువు = చిగురు + కయిదువు
గమనిక :
‘చిగురు’ అనేది ఉదంత స్త్రీ సమశబ్దం. దానికి కయిదువు అనే పదం పరమయ్యింది. కయిదువులో ‘క’ అనే పరుషం పరమైంది.
ఉదంత స్త్రీ సమశబ్దాలకు పరుషం పరమయింది కాబట్టి ‘నుగాగమం’ వచ్చింది.
ఉదా :
చిగురు + న్ + కయిదువు , తరువాత సరళాదేశం రాగా చిగురుఁగయిదువు అవుతుంది.
2. సరసపుఁదనము
జవాబు:
సరసము + తనము
గమనిక :
‘సరసముతనము’ అనేది కర్మధారయ సమాసం. అందువల్ల కర్మధారయాలలోని ‘ము’ వర్ణకానికి పు, ంపులు వస్తాయి.
ఉదా : సరసపు + తనము
ఇక్కడ పుంపులకు పరుష సరళాలు పరమైతే నుగాగమం వస్తుంది. ‘సరసపు’ లోని పుంపులకు ‘తనము’లోని ‘త’ పరుషం పరమైంది. కాబట్టి నుగాగమం (న్) వచ్చింది.
ఉదా :
సరసపు + న్ + తనము – చివరకు సరళాదేశం రాగా ‘సరసపుఁదనము’ అవుతుంది.
ఇ) నుగాగమ సంధి:
గమనిక :
ఇంకా మరికొన్ని సందర్భాల్లోనూ నుగాగమం వస్తుంది. కింది పదాలను విడదీయండి.
అ) విధాతృనానతి = విధాతృ + ఆనతి
ఆ) రాజునాజ్ఞ = రాజు + ఆజ్ఞ
విధాతృ + ఆనతి = విధాత యొక్క ఆనతి
రాజు + ఆజ్ఞ = రాజు యొక్క ఆజ్ఞ
విగ్రహవాక్యాలను అనుసరించి, పై సంధి పదాలు షష్ఠీ తత్పురుష సమాసానికి చెందినవి.
పూర్వపదం చివర స్వరాలు అనగా అచ్చులు “ఋ” కారం, “ఉత్తు” ఉన్నాయి.
గమనిక :
ఈ విధంగా షష్ఠీ తత్పురుష సమాసపదాల్లో, “A” కార, “ఋ” కారములకు అచ్చుపరమైతే నుగాగమం (5) వస్తుంది.
ఉదా :
1) విధాతృ + ఆనతి (విధాత యొక్క ఆనతి) = విధాతృ + న్ + ఆనతి
2) రాజు + ఆజ్ఞ (రాజు యొక్క ఆజ్ఞ = రాజు + న్ + ఆజ్ఞ
ఈ) నుగాగమ సంధి సూత్రం :
షష్ఠీ తత్పురుష సమాసంలో “ఉ” కార, “బు” కారాలకు అచ్చుపరమైనపుడు నుగాగమం వస్తుంది.
ఉదా :
విధాతృ + న్ + ఆనతి = విధాతృనానతి
రాము + న్ + ఆజ్ఞ = రామునాజ్ఞ
షష్ఠీ తత్పురుషంలో నుగాగమ సంధి సూత్రం :
షష్ఠీ సమాసమందు “ఉ” కార, ‘ఋ” కారాలకు అచ్చుపరమైతే నుగాగమం వస్తుంది.

పాఠ్యపుస్తకంలోని ముఖ్యమైన తెలుగు సంధులు
సంధి పదము విడదీత సంధి పేరు
1) వంటాముదము = వంట + ఆముదము = అత్వ సంధి
2) ఏమనిరి = ఏమి + అనిరి = ఇత్వ సంధి
3) అవ్విధంబున = ఆ + విధంబున = త్రిక సంధి
4) సింగపుకొదమ = సింగము + కొదమ = పుంప్వాదేశ సంధి
5) ముత్యపుచిప్ప = ముత్యము + చిప్ప = పుంప్వాదేశ సంధి
6) కొంచేపునరుడు = కొంచెము + నరుడు = పుంప్వాదేశ సంధి
7) బంధమూడ్చి = బంధము + ఊడ్చి = ఉత్వ సంధి
8) అవ్వారల = ఆ + వారల = త్రిక సంధి
9) భక్తురాలు = భక్త + ఆలు = రుగాగమ సంధి
10) బాలెంతరాలు = బాలెంత + ఆలు = రుగాగమ సంధి
11) గుణవంతురాలు = గుణవంత + ఆలు = రుగాగమ సంధి
12) దేశాలలో = దేశము + లలో = లులన సంధి
13) పుస్తకాలు = పుస్తకము + లు = లులన సంధి
14) సమయాన = సమయము + న = లులన సంధి
15) చిగురుంగయిదువు = చిగురు + కయిదువు = నుగాగమ సంధి
16) సరసపుఁదనము = సరసము + తనము = నుగాగమ సంధి
17) ఆనందాన్నిచ్చిన = ఆనందాన్ని + ఇచ్చిన = ఇత్వ సంధి
18) మేమంత = మేము + అంత = ఉత్వ సంధి
19) ఇవ్వీటిమీద = ఈ + వీటిమీద = త్రిక సంధి
20) భిక్షయిడదయ్యే = భిక్ష + ఇడదయ్యె = యడాగమ సంధి
21) ఆగ్రహముదగునె = ఆగ్రహము + తగునె = గసడదవాదేశ సంధి
22) ఉన్నయూరు = ఉన్న + ఊరు = యడాగమ సంధి
23) అందుఁ జూడాకర్ణుడు = అందున్ + చూడాకర్ణుడు = సరళాదేశ సంధి
24) చూడాకర్ణుడను = చూడాకర్ణుడు + అను = ఉత్వ సంధి
25) పరివ్రాజకుడు గలడు = పరివ్రాజకుడు + కలడు = గసడదవాదేశ సంధి
సంస్కృత సంధులు
1. సవర్ణదీర్ఘ సంధి సూత్రం
అ, ఇ, ఉ, ఋ లకు అవే అచ్చులు పరమైనపుడు వాని దీర్ఘాలు ఏకాదేశంగా వస్తాయి.
గమనిక :
‘అ’ వర్ణానికి – ‘అ’, ‘ఆ’ లు సవర్ణాలు
‘ఇ’ వర్గానికి – ‘ఇ’, ‘ఈ’ లు సవర్ణాలు
‘ఉ’ వర్గానికి – ‘ఉ’, ‘ఊ’ లు సవర్ణాలు
‘ఋ’ వర్గానికి – ‘ఋ’, ‘బూ’ లు సవర్ణాలు
1. ఉదా :
1) రామానుజుడు = రామ + అనుజుడు = (అ + అ = ఆ) = సవర్ణదీర్ఘ సంధి
2) రామాలయం = రామ + ఆలయం = (అ + ఆ = ఆ) = సవర్ణదీర్ఘ సంధి
3) గంగాంబ = గంగ + అంబ = (అ + అ = ఆ) = సవర్ణదీర్ఘ సంధి
2. ఉదా :
4) కవీంద్రుడు = కవి + ఇంద్రుడు – (ఇ’ + ఇ = ఈ), = సవర్ణదీర్ఘ సంధి
5) శ్రీకాళహస్తీశ్వరా = శ్రీకాళహస్తి + ఈశ్వర – (ఇ + ఈ = ఈ) = సవర్ణదీర్ఘ సంధి
3. ఉదా :
6) భానూదయం . = భాను + ఉదయం = (ఉ + ఉ = ఊ) = సవర్ణదీర్ఘ సంధి
7) వధూపేతుడు : వధూ + ఉపేతుడు = (ఊ + ఉ = ఊ) – సవర్ణదీర్ఘ సంధి
4. ఉదా :
8) పిత్రణం : పితృ + ఋణం = (ఋ + ఋ = ఋ) = సవర్ణదీర్ఘ సంధి
9) మాతణం = మాతృ + ఋణం = (బ + ఋ – ఋ) = సవర్ణదీర్ఘ సంధి
2. గుణ సంధి సూత్రం
అకారానికి ఇ, ఉ, ఋ లు పరమైతే, ఏ, ఓ, అర్ లు క్రమంగా ఏకాదేశంగా వస్తాయి.
1. ఉదా :
రాజేంద్రుడు = రాజ + ఇంద్రుడు = (అ + ఇ = ఏ) = గుణ సంధి
మహేంద్రుడు = మహా + ఇంద్రుడు = (ఆ + ఇ = ఏ) = గుణ సంధి
నరేంద్రుడు – నర + ఇంద్రుడు = (అ + ఇ = ఏ) = గుణ సంధి
రామేశ్వర = రామ + ఈశ్వర (అ + ఇ = ఏ) = గుణ సంధి
2. ఉదా :
పరోపకారం = పర + ఉపకారం = (అ + ఉ = ఓ) = గుణ సంధి
మహోన్నతి = మహా + ఉన్నతి = (అ + ఉ = ఓ) = గుణ సంధి
దేశోన్నతి = దేశ + ఉన్నతి = (అ + ఉ = ఓ) = గుణ సంధి
గృహోపకరణం = గృహ + ఉపకరణం = (అ + ఉ = ఓ) = గుణ సంధి
3. ఉదా :
రాజర్షి = రాజ + ఋషి = (అ + ఋ = అర్) = గుణ సంధి
మహర్షి = మహా + ఋషి = (అ + ఋ = అర్) = గుణ సంధి
పై ఉదాహరణలు పరిశీలిస్తే
1) అ, ఆ లకు ఇ, ఈ లు కలిసి ‘ఏ’ గా మారడం
2) అ, ఆ లకు ఉ, ఊ లు కలిసి ‘ఓ’ గా మారడం
3) అ, ఆ లకు ఋ, ౠలు కలిసి ‘అర్’ గా మారడం – గమనించగలం.
పై మూడు సందర్భాల్లోనూ, పూర్వ స్వరం అంటే, సంధి వీడదీసినపుడు, మొదటి పదం చివరి అచ్చులు, అ, ఆ లుగానూ, పర స్వరం, అంటే విడదీసిన రెండవ పదంలో మొదటి అచ్చులు ఇ, ఉ, ఋ – లుగానూ ఉన్నాయి.
గమనిక :
1) అ, ఆ లకు – ‘ఇ’ కలిస్తే ‘ఏ’ గా మారుతుంది.
2) అ, ఆ లకు – ‘ఉ’ కలిస్తే ‘ఓ’ గా మారుతుంది.
3) అ, ఆ లకు – ‘ఋ’ కలిస్తే ‘అర్’ గా మారుతుంది.
ఏ, ఓ, అర్ అనే వాటిని గుణాలు అంటారు. ఇలా గుణాలు వచ్చే సంధిని “గుణ సంధి” అంటారు.

3. యణాదేశ సంధి సూత్రం
ఇ, ఉ, ఋ లకు అసవర్ణాచ్చులు పరమైతే, య, వ, రలు ఆదేశంగా వస్తాయి.
ఈ కింది పదాలను విడదీయండి. మార్పును గమనించండి.
1. ఉదా :
అ) అత్యానందం = అతి + ఆనందం – (త్ + ఇ + ఆ = యా) = యణాదేశ సంధి
1) అత్యంతం = అతి + అంతం = (త్ + ఇ + అ = య) = యణాదేశ సంధి
2) అభ్యంతరం = అ + అంతరం = (త్ + ఇ + అ = య) = యణాదేశ సంధి
2. ఉదా :
ఆ) అణ్వస్త్రం = అస్త్రం (ణ్ + ఉ + అ = వ) = యణాదేశ సంధి
2) గుర్వాజ్ఞ = (ర్ + ఉ + ఆ = వ) = యణాదేశ సంధి
3. ఉదా :
ఇ) పిత్రాజ్ఞ = పితృ + ఆజ్ఞ = (ఋ + ఆ = రా) = యణాదేశ సంధి
3) మాత్రంశ = మాతృ + అంశ = (ఋ + అ = ర) = యణాదేశ సంధి
గమనిక :
ఇ, ఉ, ఋ లకు అసవర్ణాచ్చులు (వేరే అచ్చులు) పక్కన వచ్చినపుడు, క్రమంగా వాటికి య – వ -రలు వచ్చాయి. యవరలను ‘యణులు’ అంటారు. యజ్ఞులు చేరితే వచ్చే సంధిని ‘యణాదేశ సంధి అంటారు. యణాదేశ సంధిలో ‘ఇ’ కి బదులుగా “య్”, ‘ఉ’ కి బదులుగా ‘ప్’, ‘ఋ’ కి బదులుగా ‘5’ వచ్చాయి.
4. వృద్ధి సంధి సూత్రం
అకారానికి ఏ, ఐలు పరమైతే ‘ఐ’ కారమూ, ఓ, ఔ లు పరమైతే ‘ఔ’ కారమూ వస్తాయి.
ఈ కింది పదాలను విడదీయండి.
1. ఉదా :
వసుధైక = వసుధా + ఏక = = (ఆ + ఏ = ఐ) = వృద్ధి సంధి
అ) రసైక = రస + ఏక = (అ + ఏ = ఐ) = వృద్ధి సంధి
ఆ) సురైక = సుర + ఏక = (అ + ఏ = ఐ) = వృద్ధి సంధి
2. ఉదా :
సమైక్యం = సమ + ఐక్యం = (అ + ఐ = ఐ) = వృద్ధి సంధి
ఇ) అప్లైశ్వర్యం = అష్ట + ఐశ్వర్యం = (అ + ఐ = ఐ) = వృద్ధి సంధి
ఈ) దేవైశ్వర్యం =దేవ + ఐశ్వర్యం = (అ + ఐ = ఐ) = వృద్ధి సంధి
3. ఉదా :
పాపౌఘము = పాప + ఓఘము = (అ + ఓ = ఔ) = వృద్ధి సంధి
ఉ) వనౌకసులు = వన + ఓకసులు = (అ + ఓ = ఔ) = వృద్ధి సంధి
ఊ) వనౌషధి = వన + ఓషధి = (అ + ఓ = ఔ) = వృద్ధి సంధి
4. ఉదా :
రనౌచిత్యం = రస + ఔచిత్యం = (అ + ఔ – ఔ) = వృద్ధి సంధి
ఋ) దివ్యౌషధం = దివ్య + ఔషధం = (అ + ఔ – ఔ) = వృద్ధి సంధి
ఋ) దేశోన్నత్యం = దేశ + ఔన్నత్యం = (అ + ఔ – ఔ) = వృద్ధి సంధి
గమనిక :
పైన పేర్కొన్న పదాలను విడదీసినపుడు మీరు గమనింపదగిన విషయం ఇది
1. వృద్ధి సంధి ఏర్పడేటప్పుడు ప్రతిసారీ పూర్వ స్వరంగా ‘అ’.వచ్చింది.
2. పర స్వరం స్థానంలో వరుసగా “ఏ, ఏ, ఐ, ఔలు ఉన్నాయి.
3. అకారానికి ఏ, ఐలు కలిపినపుడు ‘బి’ వచ్చింది.
4. అకారానికి ఓ, ఔ లు కలిపినపుడు ‘&’ వచ్చింది.
వృద్ధులు = ఐ, ఔలను ‘వృద్ధులు’ అంటారు.
5. జశ్వ సంధి సూత్రం
పరుషాలకు వర్గ ప్రథమ ద్వితీయాక్షరాలు, శష స లు తప్ప, మిగిలిన హల్లులు కానీ, అచ్చులు కానీ, పరమైతే వరుసగా సరళాలు ఆదేశమవుతాయి.
ఉదా :
సత్ + భక్తులు = సద్ + భక్తులు = సద్భక్తులు
పై సంధి పదాలను పరిశీలించండి. మొదట విడదీసిన పదాలలోని ‘త’ కార స్థానములో, ‘ద’ కారం ఆదేశంగా వచ్చి, ‘సద్భక్తులు’ అనే రూపం వచ్చింది.
గమనిక :
ఈ విధంగా మొదటి పదం చివర, క, చ, ట, త, ప (పరుషాలు),లలో ఏదైనా ఒక అక్షరం ఉండి, రెండవ పదం మొదట క ఖ, చ ఛ, ట ఠ, త థ, ప ఫ, లు మరియు శ ష స లు తప్ప, మిగిలిన హల్లులూ, అచ్చులలో ఏ అక్షరం ఉన్నా ‘గ, జ, డ, ద, బ’ లు వరుసగా ఆదేశం అవుతాయి.
కింది పదాలను విడదీయండి.
1) దిగంతము = దిక్ + అంతము = జశ్వ సంధి (క్ – గ్ గా మారింది)
2) మృదటము = మృత్ + ఘటము = జశ్వ సంధి (త్ -ద్ గా మారింది)
3) ఉదంచద్భక్తి = ఉదంచత్ + భక్తి = జత్త్వ సంధి (త్ -ద్ గా మారింది)
4) వాగీశుడు = వాక్ ఈశుడు = జత్త్వ సంధి (క్ – గ్ గా మారింది)
5) వాగ్యుద్ధం = వాక్ + యుద్ధం = జశ్వ సంధి (క్ – గ్ గా మారింది)
6) వాగ్వాదం = వాక్ + వాదం = జశ్వ సంధి (క్ – గ్ గా మారింది)
7) తద్విధం = తత్ + విధం = జశ్వ సంధి (త్ -ద్ గా మారింది)

6. అనునాసిక సంధి సూత్రం
వర్గ ప్రథమాక్షరాలకు (కటతలకు) ‘న’ గాని, ‘మ’ గాని పరమైనప్పుడు అనునాసికములు ఆదేశంగా వస్తాయి.
ఈ కింది పదాలను విడదీయండి.
అ) వాజ్మయం = వాక్ + మయం = అనునాసిక సంధి
ఆ) రాణ్మహేంద్రవరం = రాట్ + మహేంద్రవరం = అనునాసిక సంధి
ఇ) జగన్నాథుడు = జగత్ + నాథుడు = అనునాసిక సంధి
గమనిక :
పై సంధులు జరిగిన తీరు గమనించండి.
అ) వాక్ + మయం = వాజ్మయం = ‘క్’ స్థానంలో ‘జ’ వచ్చింది.
ఆ) రాట్ + మణి = రాణ్మణి = ‘ట్’ స్థానంలో ‘ణ’ వచ్చింది.
ఇ) జగత్ + నాథుడు = జగన్నాథుడు = ‘త్’ స్థానంలో ‘న’ వచ్చింది.
గమనిక :
1) పై మూడు సంధి పదాలలోనూ మొదటి పదం చివర వరుసగా క, ట, త, లు ఉన్నాయి.
2) వాటికి ‘మ’ గాని, ‘న’ గాని పరమయినాయి. అంటే తరువాత కలిశాయి.
3) 1) అప్పుడు పూర్వపదం చివర గల ‘క’ కారం, ‘క’ వర్గకు అనునాసికమైన ‘జ’ గా మారుతుంది. (క, ఖ, గ, ఘ, ఙ)
2) అప్పుడు పూర్వపదం చివర గల ‘ట’ కారం, ‘ట’ వర్గకు అనునాసికమైన ‘ణ’ గా మారింది. (ట, ఠ, డ, ఢ, ణ)
3) అప్పుడు పూర్వపదం చివర గల ‘త’ కారం, దాని అనునాసికమైన ‘న’ (త థ ద ధ న) గా ఆదేశం అవుతాయి.
దీనినే ‘అనునాసిక సంధి’ అంటారు.
అభ్యాసము :
1) తన్మయము = తత్ + మయము = అనునాసిక సంధి
2) రాణ్మణి = రాట్ + మణి , – అనునాసిక సంధి
3) వాజ్మయము = వాక్ + మయము = అనునాసిక సంధి
4) మరున్నందనుడు = మరుత్ + నందనుడు = అనునాసిక సంధి
7.అ) విసర్గ సంధి సూత్రం
అకారాంత పదాల మీద ఉన్న విసర్గకు, వర్గ ప్రథమ ద్వితీయాక్షరాలు (క, చ, ట, త, ప, ఖ, ఛ, ఠ, థ, ఫ, శ, ష, సలు కాక, మిగతా అక్షరాలు) కలిసినపుడు, విసర్గ లోపించి “అ” కారం ‘ఓ’ కారంగా మారుతుంది.
ఉదాహరణలు చూడండి :
అ) నమోనమః = నమః + నమః
ఆ) మనోహరం : మనః + హరం
ఇ) పయోనిధి : పయః + నిధి
ఈ) వచోనియమం = వచః + నియమం
గమనిక :
ఈ నాలుగు ఉదాహరణలలో అకారాంత పదాల మీద ఉన్న విసర్గ లోపించి, ‘అ’ కారం ‘ఓ’ కారంగా మారింది.
ఆ) విసర్గ సంధి సూత్రం
విసర్గకు శ,ష,సలు కలిసినపుడు, విసర్గ శ,ష,స,లుగా మారి శ,ష,సలు ద్విత్వాలుగా మారుతాయి.
ఉదాహరణలు :
అ) మనశ్శాంతి : మనః + శాంతి
ఆ) చతుషష్టి : చతుః + షష్టి
ఇ) నభస్సుమం : నభః + సుమం
గమనిక :
విసర్గము, ప్రక్కనున్న శ, ష, స లుగా మారి, ద్విత్వాలుగా అయ్యింది. ఆయా పదాలను కలుపగా, వరుసగా మనశ్శాంతి, చతుషష్టి, నభస్సుమం అనే రూపాలు ఏర్పడ్డాయి.
ఇ) విసర్గ సంధి సూత్రం
విసర్గకు క,ఖ,ప,ఫ,లు కలిస్తే విసర్గమారదు. (సంధి ఏర్పడదు.)
ఉదాహరణలు :
అ) ప్రాతఃకాలము = ప్రాతః + కాలము = విసర్గ సంధి
ఆ) తపఃసలము = తపః + ఫలము = విసర్గ సంధి
గమనిక :
పై ఉదాహరణలలో విసర్గకు క, ఫ లు పరం అయ్యాయి. కాబట్టి విసర్గ మారకుండా యథాప్రకారంగానే ఉంది.

ఈ) విసర్గ సంధి సూత్రం
అంతః, దుః, చతుః, ఆశీః, పునః మొదలయిన పదాల తరువాత ఉండే విసర్గ, రేఫ (‘ర్’)గా మారుతుంది.
ఉదా :
అంతః + ఆత్మ : అంతర్ – + ఆత్మ = అంతరాత్మ
అ) దుః + అభిమానం = దుర్ + అభిమానం = దురభిమానం
ఆ) చతుః + దిశలు = చతుర్ + దిశలు = చతుర్దశలు
ఇ) ఆశీః + వాదము = ఆశీర్ + వాదము = ఆశీర్వాదము
ఈ) పునః + ఆగమనం = పునర్ + ఆగమనం = పునరాగమనం
ఉ) అంతః + మథనం = అంతర్ + మథనం = అంతర్మథనం
ఉ) విసర్గ సంధి సూత్రం
ఇస్, ఉర్ల విసర్గకు, క, ఖ, ప, ఫ, లు కలిస్తే, విసర్గ ‘ష’ కారంగా మారుతుంది.\
ఉదా :
ధనుష్కోటి : ధనుస్ట్ + కోటి = ధనుష్ + కోటి = ధనుష్కోటి
అ) నిష్ఫలము = నిస్ + ఫలము = నిష్, + ఫలము = నిష్ఫలము
ఆ) దుష్కరము = దుస్ + కరము – దుష్ – + కరము = దుష్కరము
గమనిక :
ఇస్ (ఇజి), ఉస్ (43) ల విసర్గలకు క,ఖ,ప,ఫ లు కలిసినపుడు, విసర్గ అనగా ‘స్’ కారము ‘ష’ కారంగా మారుతుంది.
ఊ) విసర్గ సంధి సూత్రం
విసర్గకు (అనగా ‘స్’ కు) చ ఛ లు పరమైతే ‘శ’ కారం, ట ఠలు పరమైతే ‘ష’ కారం, త థ
లు పరమైతే ‘స’ కారం వస్తాయి.
ఉదా :
అ) దుశ్చేష్టితము = దుః + చేష్టితము (విసర్గము – శ్ గా మారింది)
ఆ) ధనుష్టంకారం = ధనుః + టంకారము (విసర్గము ష్ గా మారింది)
ఇ) మనస్తాపము = మనః + తాపము (విసర్గము ‘స’గా మారింది)
ఈ) నిస్తేజము = నిః + తేజము (విసర్గము ‘స’గా మారింది)
గమనిక :
పై ఉదాహరణలలో విసర్గకు చ ఛలు పరమైతే ‘శ’ కారం, ట ఠలు పరమైతే ‘ష’ కారం త థలు పరమైతే ‘స’ కారం వస్తుంది.
పై ఉదాహరణలు గమనిస్తే విసర్గ సంధి ఆరు విధాలుగా ఏర్పడుతోందని తెలుస్తోంది.
8. శ్చుత్వ సంధి సూత్రం
‘స’ కార, ‘త’ వర్గాలకు, ‘శ’ కార ‘చ’ వర్గా (చ ఛ జ ఝ) లు పరమైతే, ‘శ’ కార ‘చ’ వర్గాలే వస్తాయి.
కింది ఉదాహరణలను పరిశీలించండి.
అ) తప + శక్తి → తపస్ + శక్తి → తపశ్శక్తి
ఆ) నిః + శంక → నిస్ + శంక → నిశ్శంక
ఇ) మనః + శాంతి → మనస్ + శాంతి → మనశ్శాంతి
గమనిక :
పై ఉదాహరణలలో పూర్వపదాలలో ఉన్న విసర్గ సంధి కార్యంలో విసర్గను ‘స’ గా తీసుకుంటున్నాం. విసర్గకు ‘స్’ కారం వస్తుంది. అలా విసర్గ స కారం కాగా, ఆ ‘స’ కారానికి ‘శ’ వర్ణం పరం అవుతుంది. ఇలా పరం అయినపుడు ఆ ‘స’ కారం, ‘శ’ కారంగా మారుతుంది. అనగా ‘శవర్ణ ద్విత్వం వస్తుంది.
ఆ) నిస్ + చింత → నిశ్చింత
సత్ + ఛాత్రుడు → సచ్ఛాత్రుడు
శరత్ + చంద్రికలు → శరచ్చంద్రికలు
జగత్ + జనని → జగజ్జనని
శార్జిన్ + జయః → శారిఞ్జయః
గమనిక :
పై పదాల్లో ‘స’ కార, ‘త’ వర్గాలు పూర్వపదాంతంగా ఉన్నాయి. ‘శ’ కార, ‘చ’ వర్గాలు (త, న) పరమైనాయి. అలా పరమైనప్పుడు ‘శ’ కార, చ వర్గాలుగా మారుతాయి.
అనగా
1) స్ + చి = శ్చి
2) త్ + జ = జ్జ
3) త్ + శా = చ్చా
4) న్ + జ = ఞ్జ
5) త్ + చ = చ్చ
ఈ విధంగా ‘స’ కార ‘త’ వర్గాలకు (తథదధన) లకు, ‘శ’ కార, ‘చ’ వర్గాలు వస్తే అది “శ్చుత్వ సంధి” అవుతుంది.

పాఠ్యపుస్తకంలోని ముఖ్యమైన సంస్కృత సంధులు
1) అత్యంత = అతి + అంత = యణాదేశ సంధి
2) పుణ్యవాసము = పుణ్య + ఆవాసము = సవర్ణదీర్ఘ సంధి
3) స్నిగ్గాంబుదము = స్నిగ్ధ + అంబుదము = సవర్ణదీర్ఘ సంధి
4) పురాతనాపాదితము = పురాతన + ఆపాదితము = సవర్ణదీర్ఘ సంధి
5) సహస్రాబ్దం = సహస్ర + అబ్దం = సవర్ణదీర్ఘ సంధి
6) వేదోక్తము = వేద + ఉక్తము = గుణ సంధి
7) మదోన్మాదము = మద + ఉన్మాదము = గుణ సంధి
8) సరభసోత్సాహం = సరభస + ఉత్సాహం = గుణ సంధి
9) జీవనోపాధి = జీవన + ఉపాధి = గుణ సంధి
10) మహోపకారం = మహా + ఉపకారం = గుణ సంధి
11) గుణౌద్ధత్యం = గుణ + ఔద్ధత్యం = వృద్ధి సంధి
12) రసైకస్థితి = రస + ఏకస్థితి = వృద్ధి సంధి
13) తన్మయము = తత్ + మయము = అనునాసిక సంధి
14) వాజ్మయము = వాక్ + మయము = అనునాసిక సంధి
15) రాణ్మణి = రాట్ + మణి = అనునాసిక సంధి
16) మరున్నందనుడు = మరుత్ + నందనుడు = అనునాసిక సంధి
17) రాణ్మహేంద్రపురం = రాట్ + మహేంద్రపురం = అనునాసిక సంధి
18) జగన్నాథుడు = జగత్ + నాథుడు = అనునాసిక సంధి
19) నమోనమః = నమః . + నమః = విసర్గ సంధి
20) మనోహరం = మనః . + హరం = విసర్గ సంధి
21) పయోనిధి = పయః + నిధి = విసర్గ సంధి
22) వచోనిచయం = వచః + నిచయం = విసర్గ సంధి
23) ప్రాతఃకాలము = ప్రాతః + కాలము = విసర్గ సంధి
24) తపఃఫలము = తపః + ఫలము = విసర్గ సంధి
25) నిష్ఫలము = నిస్ + ఫలము = విసర్గ సంధి
26) దుష్కరము = దుస్ + కరము = విసర్గ సంధి
27) ధనుష్టంకారము = ధనుః + టంకారము = విసర్గ సంధి
28) మనస్తాపము = మనః + తాపము = విసర్గ సంధి
29) దురభిమానం = దుః + అభిమానం = విసర్గ సంధి
30) నిరాడంబరం = ఆడంబరం = విసర్గ సంధి
31) దుర్భేద్యము = దుః + భేద్యము = విసర్గ సంధి
32) తపోధనుడు = తపః + ధనుడు = విసర్గ సంధి
33) నిరాశ = నిస్ + ఆశ = విసర్గ సంధి
34) దుశ్చేష్టితము = దుస్ + చేష్టితము = శ్చుత్వ సంధి
35) నిశ్చింత = నిస్ + చింత = శ్చుత్వ సంధి
36) సచ్ఛాత్రుడు = సత్ + ఛాత్రుడు = శ్చుత్వ సంధి
37) శరచ్చంద్రికలు = శరత్ + చంద్రికలు = శ్చుత్వ సంధి
38) జగజ్జనని = జగత్ + జనని = శ్చుత్వ సంధి
39) శారిజ్జయః = శార్జిన్ + జయః = శ్చుత్వ సంధి
![]()
![]()
![]()
![]()