Students can go through AP State Board 10th Class Physical Science Notes Chapter 11 Principles of Metallurgy to understand and remember the concept easily.
AP State Board Syllabus 10th Class Physical Science Notes Chapter 11 Principles of Metallurgy
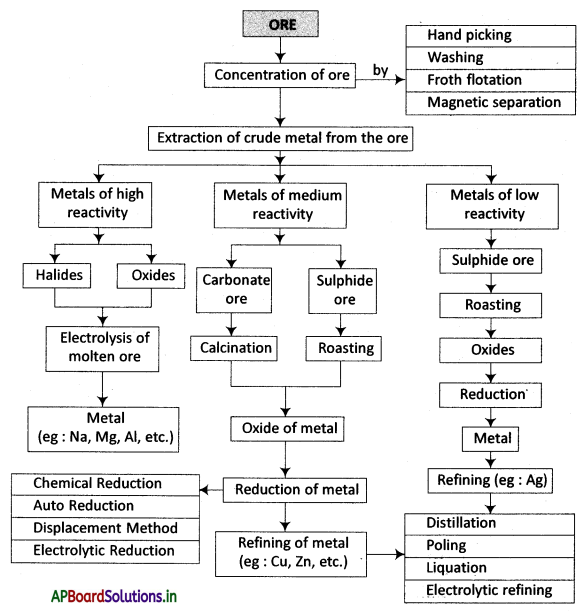
→ Metallurgy is the process of extraction of metals from their ores and the preparation of alloys.
→ Metals like gold (Au), silver (Ag) and copper (Cu) are available in nature in a free state as they are least reactive.
→ The elements or compounds of the metals which occur in nature in the earth’s crust are called minerals.
→ The minerals from which the metals are extracted without economical loss are called ores.
→ The removal of unwanted rocky material from ore before it converts into metal is called concentration.
→ Air under pressure is blown to produce froth in water is called froth flotation.
→ If the ore or impurity, one of them is magnetic and the other is non-magnetic, they are separated using electromagnets.
→ Arranging the metals in decreasing order of their reactivity is known as activity series.
→ Extraction of the metals at the top of the activity series is the electrolysis of their fused compounds.
→ Sulphide ores are converted into oxides by heating them strongly in excess of air. This process is known as “roasting”.
![]()
→ When sulphide ore of copper is partially roasted, the rest of the sulphide ore reacts with oxide and forms the metal. This is called auto reduction.
→ In displacement reactions, a more active metal displaces a less active metal from its compound.
→ Displacement reactions are highly exothermic.
→ The reaction of iron (III) oxide (Fe2O3), with aluminium, is used to join railings of railway tracks or cracked machine parts. This reaction is known as the thermite reaction.
→ Metals at bottom of the activity series are often found in a free state.
→ The process of obtaining the pure metal from the impure metal is called refining of the metal.
→ The impurities are removed either as gases or get oxidized and form scum (slag) over the surface of the molten metal. This is called poling.
→ Blister copper is purified by poling.
→ Distillation is very useful for the purification of low boiling metals like zinc and mercury-containing high boiling metals as impurities.
→ Metals are not available as free elements on earth.
→ Human history in terms of materials had the Bronze age and Iron age. Pertaining to metals, they started to use bronze and iron.
→ Bauxite ore contains 50-70% aluminium oxide.
→ Ores of many metals are oxides.
→ The impurities of ore are called gangue.
![]()
→ The concentration of ore can be done in 4 methods.
- Handpicking
- Washing
- Froth flotation
- Magnetic separation.
→ The only viable method to extract metals like K, Na, Ca, Mg and Al is electrolysis.
→ Minerals: The elements or compounds of the metals which occur in nature in the earth’s crust are called minerals.
→ Ores: The minerals from which the metals are extracted without economical loss are called ores.
→ Froth flotation: This method is mainly useful for sulphide ores which have no wetting property whereas the impurities get wetted.
→ Thermite process: The reaction between metal oxides and aluminium.
→ Distillation: Separation of low boiling metals like zinc and mercury from high boiling metals as impurities.
→ Poling: The molten metal is stirred with logs of greenwood.
→ Liquation: A low melting metal like a tin can be made to flow on a soapy surface to separate it from high melting impurities.
→ Electrolytic refining: The electrolysis process is used to purify the metal.
→ Smelting: Smelting is a pyrochemical process in which the ore is mixed with flux and fuel and then strongly heated.
→ Roasting: Sulphide ores are converted into oxides by heating them strongly in excess of air. This process is known as roasting.
→ Calcination: Calcination is a pyrochemical process in which the ore is heated in the absence of air.
→ Blast furnace: Furnace used for smelting.
→ Reverberatory furnace: Furnace used for roasting.
→ Metallurgy: It is the process of extraction of metals from their ores and the preparation of alloys.
![]()
→ Gangue: The impurities obtained from ore like days are called gangue.
→ Activity series: Arranging the metals in decreasing order of their reactivity is known as activity series.
→ Thermite reaction: The reaction of iron (III) oxide (Fe2O3), with aluminium, is used to join railings of railway tracks or cracked machine parts. This reaction is known as a thermite reaction.
→ Refining of the metal: The process of obtaining the pure metal from the impure metal is called refining of the metal.
→ Corrosion: Metallic objects are slowly coated with oxides or other salts of the metal and form a thin layer which is called corrosion.
→ Rusting: Corrosion of iron is called rusting.
→ Flux: Flux is a substance added to the ore to remove the gangue from it by reacting with ore.
→ Furnace: Furnace ¡s the one which is used to carry out pyrochemical processes in metallurgy.
→ Hearth: The hearth is the place inside the furnace where the ore is kept for heating.
→ Chimney: Chimney is the outlet through which flue (waste) gases go out of the furnace.
→ Concentration or Dressing: concentration is the removal of the unwanted rocky material as possible before the ore is being converted into metal.
→ Auto (self) reduction: When sulphide ore of copper is partially roasted it gives oxide when the supply of air is stopped, the rest of sulphide reacts with oxide and forms the metal and SO2 is called auto reduction.
![]()
→ Displacement reactions: The reaction in which a more active metal displaces less active metal from its compound is called displacement reaction.
→ Firebox: Firebox is the part of the furnace where the fuel is kept for burning.
→ Sir Henry (1813 – 1898):
- Bessemer Sir Henry was born in Chariton on January 19, 1813.
- He invented a new process for the metallurgy of steel, which became quite famous.
- He Invented a device named after him as “Bessemer Converter” and the phenomenon is called Basscmerisation”.