Students can go through AP State Board 7th Class Science Notes Chapter 2 Acids and Bases to understand and remember the concept easily.
AP State Board Syllabus 7th Class Science Notes Chapter 2 Acids and Bases
→ Fruits, vegetables and other food substances have different tastes.
→ Those substances that indicate a change in colour when some substances are added to them are known as indicators.
→ The substances which are soapy to touch are basic in nature.
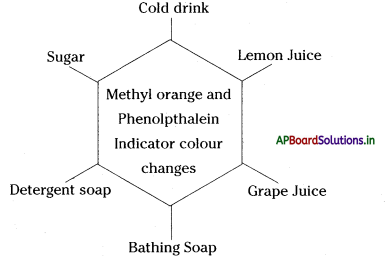
→ Methyl orange gives a red colour with acids and yellow colour with bases.
→ Phenolphthalein remains colourless in acidic solution while it turns pink in basic solution.
→ Neutral solutions have no effect on Indicators.
→ When acids and bases are mixed in definite proportions they give a neutral solution. Salts that change blue litmus to red are acidic salts.
→ Salts that change red litmus to blue are basic salts.
→ The indicator helps us to find whether the solution is acidic or basic or neutral.
→ Red litmus paper, blue litmus paper, phenolphthalein, methyl orange, Hibiscus, turmeric and rose paper are natural indicators.
![]()
→ The substances that turn blue litmus to red are acidic in nature.
→ The substances that are soapy to touch and turn red litmus to blue are basic in nature. Acid rains are the combination of Carbonic acid, Sulphuric acid and Nitric acid with rainwater.
→ All substances whose solutions are neutral are not salts. For eg., sugar or starches give neutral solutions but they are not salts.
→ In the process of Neutralization, both the acidic and basic qualities are destroyed.
→ Salts need not always be neutral. They can also be acidic or basic.
→ Indicator: A substance used in titrations to indicate the completion of a chemical reaction, usually by a change of colour a substance, such as litmus, that indicates the presence of an acid or alkali.
→ Acid: A compound usually having a sour taste.
→ Base: A compound usually having a bitter taste.
→ Red litmus: Litmus, an organic dye usually used in the laboratory as an indicator of acidity or alkalinity Naturally pink in colour, it turns blue in alkali solution.
→ Blue litmus: Litmus, an organic dye usually used in the laboratory as an indicator of acidity or alkalinity. Naturally pink in colour, it turns blue in alkali solution.
→ Acidic substances: The substances that contain different acids and the ability to turn blue litmus red. Ex: lemon – citric acid, banana – ascorbic acid etc.
→ Basic substances: The substances which are soapy to touch are basic in nature. Ex: soap, glass cleaner, etc.
![]()
→ Neutral substance: A neutral substance is a material that exhibits neither Acid nor a Base property and does not change the colour of litmus paper.
→ Salts: A chemical compound formed by replacing all or part of the hydrogen ions of an acid with metal ions or electropositive radicals.
→ Neutralization: A reaction between an acid and a base that yields salt and water.
→ Acid rain: Rain that contains a high concentration of pollutants, chiefly sulphur dioxide and nitrogen oxide, released into the atmosphere by the burning of fossil fuels such as coal or oil.
→ Caustic soda: A strongly alkaline caustic soda used in manufacturing soap and paper and aluminium and various sodium compounds.
→ Vinegar: A sour-tasting liquid consisting of impure dilute acetic acid, made by oxidation of the ethyl alcohol in beer, wine, or cider, It is used as a condiment or preservative.
→ Fire extinguisher: A portable apparatus containing chemicals that can be discharged in a rapid stream to extinguish a small fire.
→ Why are the inner sides of vessels made up of brass and copper coated?
When some substances are kept in a copper container for a long time then a blue-green layer is formed in the inner walls of the container. Copper reacts with the acids present in the substances and forms a blue-green compound. To avoid this reaction the inner wálls of these vessels are coated with Tin.
![]()
→ Robert Koch: Robert Koch was born on 11th December 1843 in Kaushal, a village in the mountainous terrain of the Harz in the state of Hannover in Germany. His boyhood days were spent in an atmosphere of poverty, struggle and hope – all mingled in mixed proportions, yet with a happy turnover, on the overall.
At the age of 23, Koch qualified for M.D., the Laudable qualification heralding the future genius in him. Koch formulated the symptoms and preventive measures of tuberculosis and cholera. Koch is respected all over the world. He was the recipient of the Nobel prize for Medicine in 1905. The title ‘Excellenz’ was awarded to him. outstanding contribution- in tuberculosis.