SCERT AP 7th Class Science Study Material Pdf 10th Lesson Changes Around Us Textbook Questions and Answers.
AP State Syllabus 7th Class Science 10th Lesson Questions and Answers Changes Around Us
7th Class Science 10th Lesson Changes Around Us Textbook Questions and Answers
Improve Your Learning
I. Fill in the blanks.
1. Changes in which new substances are formed are called ______ changes.
2. Magnesium + Oxygen → ______
3. Milk converted to curd is ______ change.
4. Rusting of iron is protected by ______ process.
Answer:
1. Chemical
2. Magnesium Oxide
3. Chemical
4. Galvanisation
II. Choose the correct answer.
1. When a woolen yarn is knitted to get a sweater, the change can be described as
a) Physical change
b) Chemical change
c) Endothermic reaction
d) Exothermic reaction
Answer:
a) Physical change
2. The chemical chancre among the following is ______
a) Water to clouds
b) GroWth of a tree
c) Cow dung to bio gas
d) Ice to water
Answer:
b) GroWth of a tree
3. Which is an example of a periodic change?
a) Earth quake
b) Formation of rainbow
c) Occurrence of tides in sea
d) Showering of rain
Answer:
c) Occurrence of tides in sea
![]()
4. Photosynthesis by green plant is a
a) Physical change
b) Chemical change
c) Reversible change
d) None of the above
Answer:
b) Chemical change
III. Matching.
| Group – A | Group – B |
| A) Growing hair | 1. Chemical change |
| B) Breaking Mirror | 2. Acetic Acid |
| C) Galvanisation | 3. Slow change |
| D) Vinegar | 4. Physical change |
| E) Atmospheric pollution | 5. Depositing zinc on iron metal |
| 6. Fast change |
Answer:
| Group – A | Group – B |
| A) Growing hair | 3. Slow change |
| B) Breaking Mirror | 4. Physical change |
| C) Galvanisation | 5. Depositing zinc on iron metal |
| D) Vinegar | 2. Acetic Acid |
| E) Atmospheric pollution | 1. Chemical change |
IV. Answer the following questions.
Question 1.
Distinguish physical and chemical changes.
Answer:
| Physical Changes | Chemical Changes |
| 1) No new substances are formed. | 1) During this new substances are formed. |
| 2) The chemical properties of a sub-stance do not change. | 2) The chemical properties of a substance changes. |
| 3) It is temporary and reversible in nature. | 3) It is permanent and irreversible change. |
| 4) Change in the properties such as colour, shape and size of a sub-stance occurs. | 4) A colour change may take place and sound may be produced. Heat or light may be ab¬sorbed or released. |
Question 2.
When a candle is burnt, what type of changes takes place? Give another example of the similar process.
Answer:
- When a candle is burnt both physical and chemical changes take place.
- Burning of candle giving heat and light and reducing its size is a chemical change.
- The wax melted during burning again condenses to solid wax is a physical change.
- Part of the candle burnt is a permanent change and it cannot be reversed.
- Due to the burning of the candle, carbon dioxide and water vapour are produced.
Examples:
- Burning of LPG in our kitchen.
- Liquid state of LPG in the cylinder is converted into gaseous state when comes out This is a physical change.
- When LPG burns in air heat is produced, this is a chemical change.
Question 3.
Define crystallization.
Answer:
The process of separating a soluble solid from the solution by heating or evaporating is called crystallization.
Question 4.
Guess the consequences of burning crackers during festivals and celebrations.
Answer:
- Firing crackers during festivals and celebrations increase the concentration of dust and pollutants in the air.
- The dust particles get settled on the surrounding surfaces which are packed with chemicals like copper, zinc, sodium and magnesium. They cause damage to paintings.
- The quality of ambient air will be decreased drastically.
- Firing of crackers causes lot of sound pollution.
- The oxides of sulphur pollute the air and causes lung diseases to human beings.
- The increase in levels of air pollutants cause acid rains, corrosion of objects and decrease in visibility.
Question 5.
Prove experimentally chemical change is a permanent change.
Answer:

Aim : To prove chemical change is a permanent change.
Materials required :
Magnesium ribbon, water, match box, bunsen flame.
Procedure:
1) Take a small piece of Magnesium ribbon.
2) Burn it on a flame we will find brilliant white dazzling light leaving a powdery substance behind.
3) This is because when magnesium burns in the presence of oxygen, it forms magnesium oxide in the form of powdered ash. This is a new substance.
Magnesium + Oxygen → Magnesium Oxide (new substance)
This is a permanent change.
4) Now, take a small quantity of Magnesium oxide and mix it with a small quantity of water and dissolve it.
Magnesium Oxide + Water → Magnesium Hydroxide (new substance)
This is also a permanent change.
Here also another new substance is formed.
5) By testing this new substance with blue and red litmus papers. Red litmus turns blue. Then Magnesium Hydroxide is a base.
Inference :
Changes that occur with the formation of new substance with different chemical Coposition or transformation of a substance into another substance with the evolution or absorption of heat; or light energy are termed as chemical changes. These are permanent changes.
![]()
Question 6.
Guess the reasons for increase of plastic pollution.
Answer:
Main reasons for increase of plastic pollution :
- Rising human population and their needs, people depend on plastic.
- The food industry which packs everything on plastic.
- Increase in usage of plastic bottles and container caps.
- Indiscriminate usage of plastic bags and carriers.
- Over usage of plastic straws and stirrers.
- Lack of proper management of plastic waste.
- Unawareness among the public about the hazardous affects of plastic wastes.
- People are not following the 3’Rs (Recycle – Reduce – Reuse) method to reduce plastic wastes.
Question 7.
How can you appreciate the role of periodic changes in nature?
Answer:
- Changes that are repeating at regular intervals of time are called as periodic changes.
- Formation of day and night, for every 12 hours the sun rise and the sun set are repeating.
- Seasons like summer, rainy, winter and spring occur every year at regular intervals.
- The full moon and new moon repeats every month.
- Heart beat of human beings takes place periodically.
- Low and high tides in sea are periodic.
- All these changes are periodically taking place in nature.
- They provide us all comforts to live on this earth. I appreciate these periodic changes in nature for providing a happy life on this earth.
- Without these changes in environment, life of us would become impossible.
Question 8.
Suggest some methods to prevent rusting of iron articles.
Answer:
- The problem of rusting of iron and other metal articles is common experience in almost every home.
- It spoils beautiful articles and makes them look ugly.
- The following are some of the ways to prevent the rusting of iron.
a) Do not allow the iron articles to come in direct contact with Oxygen in the air, water or both.
b) Apply a coat of paint or grease on an iron article.
Question 9.
Ravi prepared carbon-dioxide using baking soda and vinegar. Carbon-dioxide changed lime water into milky white. Represent this experiment in a diagram with labelling.
Answer:
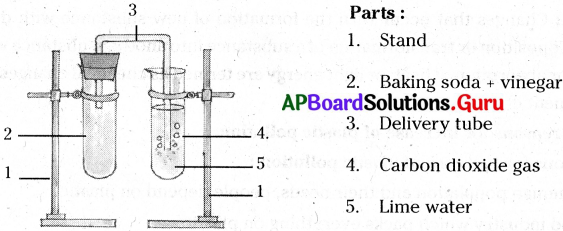
Preparation of carbon dioxide
7th Class Science 10th Lesson Changes Around Us InText Questions and Answers
7th Class Science Textbook Page No. 54
Question 1.
What change do you observe after removing mehandi applied on your palms?
Answer:
The hands get red colour after removing mehandi.
Question 2.
Can we clear the printed letters on paper. Why?
Answer:
No, it is a permanent change.
![]()
Question 3.
Water dispenser gives hot, normal and cold water. Is this natural or man made?
Answer:
Man-made.
7th Class Science Textbook Page No. 55
Question 4.
What is change?
Answer:
An act or process through which something becomes different.
Question 5.
How do we know that something has changed?
Answer:
By observing the changes in shape, size, colour and chemical properties.
Question 6.
What are the possible reasons for that change?
Answer:
They may be because of physical or chemical changes.
Question 7.
Is there any involvement of human beings?
Answer:
No, these are natural changes.
7th Class Science Textbook Page No. 56
Question 8.
Is there any change in its shape?
Answer:
Yes, it’s size will increase.
Question 9.
How does it happen?
Answer:
The air blown into the balloon occupies the space and the balloon becomes bigger in size. ,
Question 10.
Is it by itself or by anybody else?
Answer:
It is a man made change.
![]()
Question 11.
So what type of change it is?
Answer:
This is a man made change.
Question 12.
How much time they require?
Answer:
They require longer periods. It is a slow process.
7th Class Science Textbook Page No. 57
Question 13.
Have you ever noticed the following changes in our daily life?
Answer:
Yes. It is also a reversible change.
7th Class Science Textbook Page No. 58
Question 14.
Do the formed substance differs from original substance?
Answer:
Yes. The formed new substance is completely different.
Question 15.
Can we get original substance by reversing the conditions?
Answer:
No.
7th Class Science Textbook Page No. 50
Question 16.
Have you seen large crystals of sugar (Missrf) or crystal salt?
Answer:
Yes.
Question 17.
Do you know how we get these crystals?
Answer:
Yes. We get them through crystallization.
Question 18.
Have you ever observed the formation of small sugar crystals on sweets like Jilebi and badushah, which are kept aside for a long period?
Answer:
Yes.
![]()
Question 19.
What is the reason for this?
Answer:
It is because of crystallization.
Question 20.
What changes do you notice at the end?
Answer:
We notice the formation of large size crystals of sugar at the bottom of the beaker.
Question 21.
Do you find any crystals in the solution?
Answer:
No, we will find the crystals at the bottom of the beaker.
Question 22.
What type of change it is?
Answer:
It is a physical change.
7th Class Science Textbook Page No. 62
Question 23.
In Which cases new substances are formed?
Answer:
In chemical changes, we get new substance.
Question 24.
Have you observed rusting of iron, curdling of milk?
Answer:
Yes, I observed many times.
Question 25.
Do we get the same substance after change? Is the change is temporary or permanent?
Answer:
No, it is a permanent change.
Question 26.
Does the ash formed look like Magnesium ribbon?
Answer:
No.
Question 27.
Do you think the magnesium ribbon and the ash have the same composition?
Answer:
No
Question 28.
What do you observe?
Answer:
We get Magnesium Hydroxide.
![]()
Question 29.
Do you observe any change in the state of the substance?
Answer:
It is in liquid state (from solid state)
Question 30.
Is it an acid or base?
Answer:
Base.
7th Class Science Textbook Page No. 63
Question 31.
Did you observe iron nails, iron gates, iron benches or pieces of iron left in the open ground for a long time?
Answer:
Yes.
Question 32.
Did you observe any change in colour?
Answer:
We can observe a brown colour layer on the surface of iron articles.
Question 33.
How can we protect the iron articles from rusting?
Answer:
Through a process called Galvanization.
7th Class Science Textbook Page No. 64
Question 34.
Are there any other ways by which rusting of iron can be prevented?
Answer:
When the iron articles are coated with metals like chromium or zinc, we can prevent them from rusting.
Question 35.
Have you observed the handles of bicycles, metal rims of bicycle and motor cycles?
Answer:
Yes.
Question 36.
Do these articles rust? If not why?
Answer:
No, because they are coated with zinc or chromium.
Question 37.
What changes do you notice?
Answer:
We can notice a brown layer on their outer surface.
Question 38.
Can you prevent the browning of cut vegetables and fruits?
Answer:
Yes.
Question 39.
Have you observed your mother keeping the cut potatoes or brinjals in cold water?
Answer:
Yes.
7th Class Science Textbook Page No. 65
Question 40.
In which fruit or vegetable do you notice change in colour?
Answer:
In Apple, Brinjal and Potato.
Question 41.
Why does this change occurs?
Answer:
Because some vegetables and fruits react with oxygen in the air when they cut. Due to this oxidation process, brown layer is formed on the surface of these fruits and vegetables.
Think & Respond
Question 1.
When food gets spoiled, it produces a foul smell. Shall we call this change as a chemical change?
Answer:
When food gets spoiled by micro organisms, it produces a foul smell. By the action of the enzymes released by these microbes, the components of the food undergo chemical changes. Due to this, the food emits foul smell. It is a permanent change and irreversible in nature.
![]()
Question 2.
You know that plants produce their food by a process called photosynthesis. Can we call photosynthesis a chemical change?
Answer:
Photosynthesis is a chemical change. Here, carbondioxide is reduced to glucose. In this process oxygen is evolved. It is a permanent and irreversible change. New substance are formed.
Question 3.
Do all the materials react with oxygen in the air?
Answer:
No.
Question 4.
Observe Gold and Silver. You wear them in the form of ornaments. Even if they get exposed to air for long time, they do not change their colour. Why?
Answer:
Metals like gold and silver, even if they are exposed to air for a long time, they do not change colour or get rusted.
It means that they are resistant to corrosion which is the reason why we use them in making ornaments.
Activities and Projects
Question 1.
Collect information on the process of artificial ripening of fruits in fruit market and discuss whether it is useful or harmful.
Answer:
- Calcium carbide more commonly known as “masala” is used for artificial ripening of fruits. It is very harmful to health.
- Calcium carbide is a carcinogenic agent.
- The most important precaution to avoid eating such artificially ripened fruits is to go in for fruits and vegetables which are not unseasonal.
- Always wash the vegetables and fruits properly before consuming.
Question 2.
When you burn a piece of wood different changes take place analyse the following. Predict possible changes and list them all.
a) Are there any physical changes among them?
b) How many forms of energy are released in the change?
c) What chemical changes do you notice?
d) Explain briefly why these occur.
Answer:
a) No physical changes is observed.
b) The energy is released in the form of heat sound and light energy.
c) When a piece of wood is burnt a new material is formed (ash). We also notice change in shape and size of new materials (powder). This type of change which leads to form new substance is called chemical changes.
d) Wood turns into carbondioxide and ash
Carbon (Wood) + Oxygen (Air) → CO2 + Ash
Activities
Activity – 1
Question 1.
What changes do you observe, When a balloon is blown?
Answer:
Change in the shape of balloon is done by blowing air into it. This is a man made change. When we left off the air from it, it returns to its normal shape. This is a physical change and reversible one.
Activity – 2
Question 2.
Observe the changes mentioned in the following table and complete it.
| Change | Time taken shorter/longer duration |
Type of change Fast/ Slow |
| 1. Digestion of food | ||
| 2. Burning a small candle | ||
| 3. Occurrence of lightening | ||
| 4. Construction of Dam | ||
| 5. Rusting of iron |
Answer:
| Change | Time taken shorter/longer duration |
Type of change Fast/ Slow |
| 1. Digestion of food | Longer duration | Slow |
| 2. Burning a small candle | Shorter | Fast |
| 3. Occurrence of lightening | Shorter | Fast |
| 4. Construction of Dam | Longer duration | Slow |
| 5. Rusting of iron | Longer duration | Slow |
Activity – 3
Question 3.
What changes do you notice when few pieces of ice in a beaker are heated? What do you mean by a physical change? Before heating After heating After freezing
Answer:
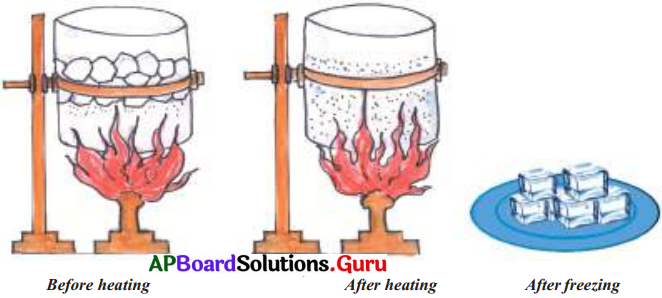
- Take few pieces of ice in a beaker and heat them on burner as shown in the figure.
- We notice that ice slowly melts and becomes water and on further heating it changes to steam.
- If we reduce the temperature, the water vapour changes back to water and when temperature is further reduced it changes to ice.
- In the above activity we notice the change of the state of ice to water and to vapour but the substance, water, remains the same.
- Changes of this type where no new substance is formed are known as physical changes.
- When a material undergoes a change in shape, size, colour or state it is called a Physical Change.
- Generally, no new substance is formed in a physical change.
Activity – 4
Question 4.
Describe how do you perform the activity to observe the reaction of vinegar with baking soda?
Answer:
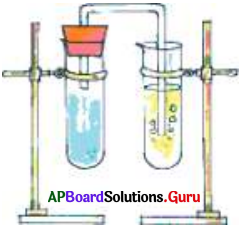
- First set up the apparatus as shown in Fig.
- Take a teaspoon of vinegar (acetic acid) in a test tube and add a pinch of baking soda (Sodium bicarbonate) to it.
- You observe bubbles coming out with a hissing sound. Pass this gas through freshly prepared Limewater (Calcium Hydroxide).
- Limewater changes to milky white showing that the gas sent into the test tube is Carbon dioxide.
- Vinegar + Baking Soda → Sodium acetate + Carbon dioxide + Water
- Carbon dioxide + Lime Water → Calcium Carbonate + Water
- In these reactions the new substances like Carbon dioxide and Calcium Carbonate are formed. Hence it is a chemical change.
- When a material undergoes a change in its composition it is called a chemical change.
![]()
Activity – 5
Question 5.
Write the approximate time after which they repeat.
| Natural | Period of time of repetetion (Approximate) |
| 1. Change of day and night | Every 12 hours |
| 2. Withering of leaves | |
| 3. Rising of the pole star | |
| 4. Change of Seasons | |
| 5. Appearance of full moon |
Answer:
| Natural | Period of time of repetetion (Approximate) |
| 1. Change of day and night | Every 12 hours |
| 2. Withering of leaves | 1 year |
| 3. Rising of the pole star | 24 hours |
| 4. Change of Seasons | 1 year |
| 5. Appearance of full moon | 30 days |
Activity – 6
Question 6.
How do you produce large size of sugar crystals ? What type of change is it?
Answer:
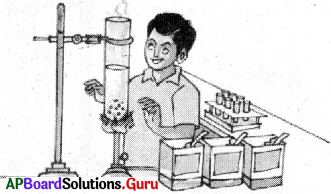
- Take a big size test tube. Fill half of it with water. Add some sugar to it and stir it.
- Keep adding sugar and stirring until saturation is attained.
- Then heat this sugar solution and add some more sugar to it while stirring continuously.
- Continue adding sugar till no more sugar can be dissolved in it.
- Now filter the solution and allow it cool for half an hour.
- We notice formation of large size crystals of sugar at the bottom of the beaker. Thus the small granules of sugar added changed into large size sugar crystals.
- This is a physical change.
Activity – 7
Question 7.
Some changes are given in the table. Write possible changes you notice for each case and put (✓) in the appropriate column.
Answer:
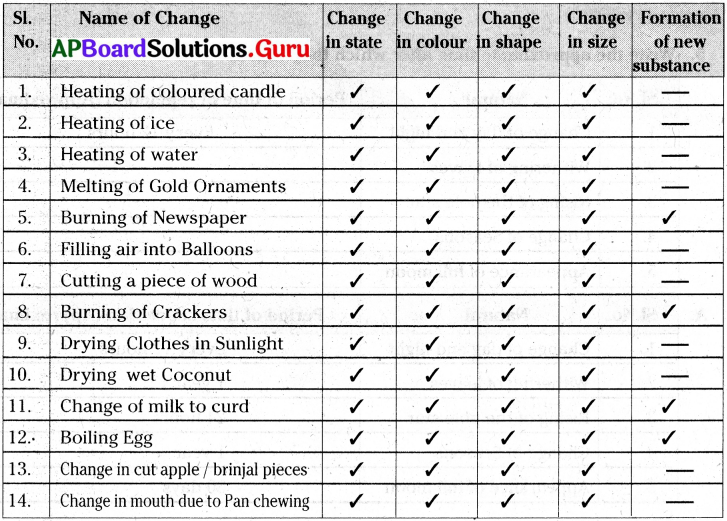
1) In the above activity we notice that only in some examples like burning of paper, burning of crackers, change of milk to curd, boiling of egg, etc., a new substance is formed.
2) But in other examples of changes we notice an change in state or colour or size or shape etc but the substance remains same and no new substance is formed.
Activity – 9
Question 9.
Complete the following table.
| Name of the Fruit | Whether turned brown or not | |
| Yes | No | |
| 1. Apple | ||
| 2. Brinjal | ||
| 3. Potato | ||
| 4. Tomato | ||
| 5. Cucumber | ||
| 6. Mango | ||
Answer:
| Name of the Fruit | Whether turned brown or not | |
| Yes | No | |
| 1. Apple | ✓ | |
| 2. Brinjal | ✓ | |
| 3. Potato | ✓ | |
| 4. Tomato | ✓ | |
| 5. Cucumber | ✓ | |
| 6. Mango | ✓ | |
Some fruits and vegetables when cut, react with oxygen in the air. The process of reaction with oxygen is called oxidation. Due to this process brown layer is formed on the surface of fruits and vegetables.