Students can go through AP State Board 8th Class Physical Science Notes Chapter 9 Electrical Conductivity of Liquids to understand and remember the concept easily.
AP State Board Syllabus 8th Class Physical Science Notes Chapter 9 Electrical Conductivity of Liquids
→ Some liquids also conduct electricity as some solids do.
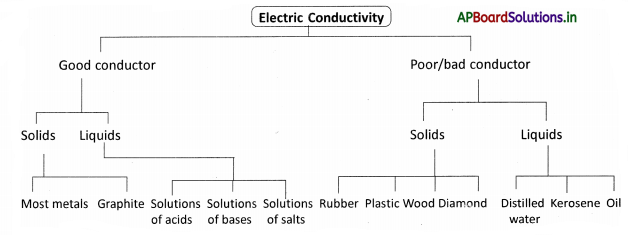
→ The materials which allow an electric current to pass through them easily are called good conductors of electricity.
→ The materials that do not allow current to pass through them are called bad or poor conductors of electricity.
→ Some liquids are good conductors of electricity and some are poor conductors. Distilled water does not allow the current to pass through it.
→ Rainy water and water used at homes is a good conductor of electricity due to dissolved salts.
→ Most liquids that conduct electricity are solutions of acids, bases, salts. The electrolyte is a solution of a substance through which electric current can pass. The process of decomposition of the solution of the compound by passing electricity is called electrolysis.
![]()
→ The metallic rods or plates used for electrolysis are called electrodes.
→ The process of depositing a layer of any desired metal on another material by means of electricity is called electroplating.
→ The passage of an electric current through a conducting liquid causes chemical reactions. The resulting effects are called chemical effects of current.
→ An electrolytic cell is a device that converts chemical energy into electrical energy.
→ Good conductors: The materials which allow an electric current to pass through them easily are called good conductors of electricity.
→ Bad conductors or: The materials which do not allow passage of current through poor conductors them areca lied poor conductors or bad conductors of electricity.
→ Electrodes: The metallic rods or plates used in the electrolysis process are called electrodes.
→ Electrolyte: Electrolyte is a solution of a substance through which electric current can pass.
→ Electrolysis: The process of decomposing a solution of the compound into constituent elements or ions by passing electricity is called electrolysis.
→ Electroplating: Electroplating is a process in which one metal ¡s coated with another metal by using an electrolysis process.
→ Electrolytic cell: Electrolytic cell ¡s a device that converts chemical energy into electric energy.
→ Volta cell: First primary cell made by Volta.
→ LED: Light Emitting Diode. The diode glows when the current passes through it.
![]()
→ Key: This connects or disconnects an electrical circuit.
→ Corrosion: Decay of a metal due to reaction with air, moisture, and carbon dioxide.
→ William Nicholson (1753 – 1815):
- He was a British chemist
- He conducted many experiments on the chemical effects of current
- In 1800 he had shown that if electrodes were immersed in water, and a current was passed, bubbles of oxygen and hydrogen were produced.
- Oxygen bubbles formed on the electrode connected to the positive terminal of the battery and hydrogen bubbles formed on the other electrode.