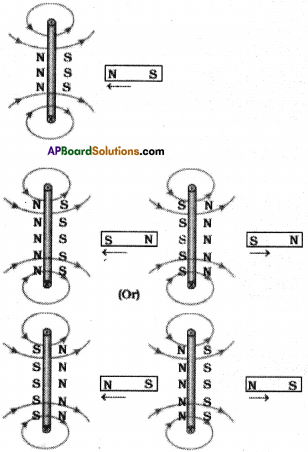
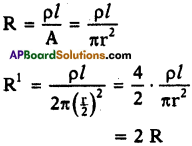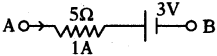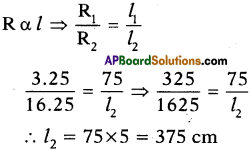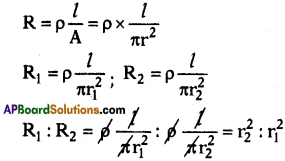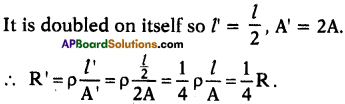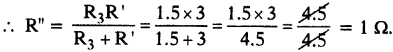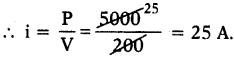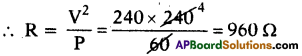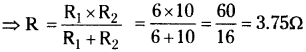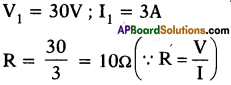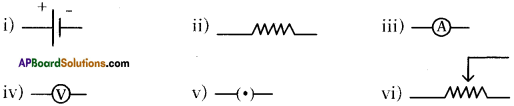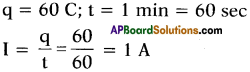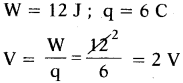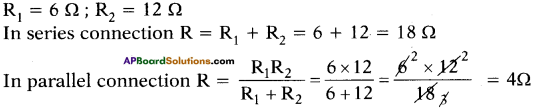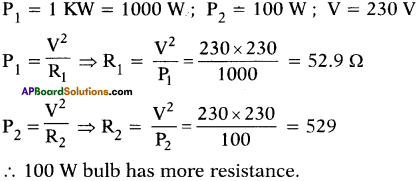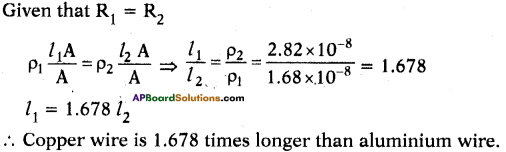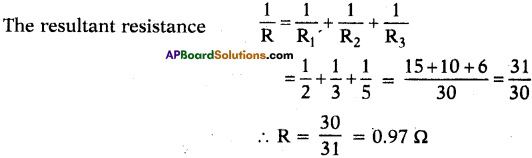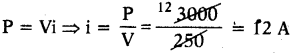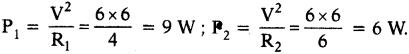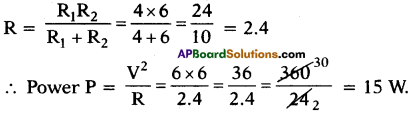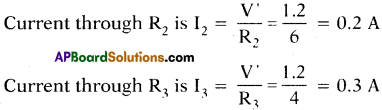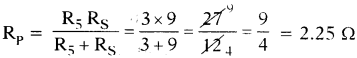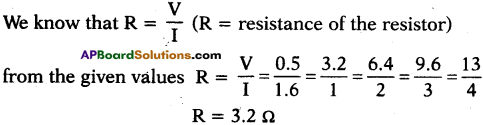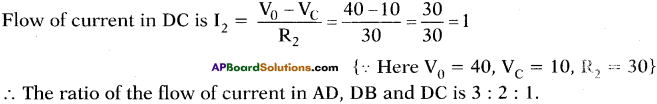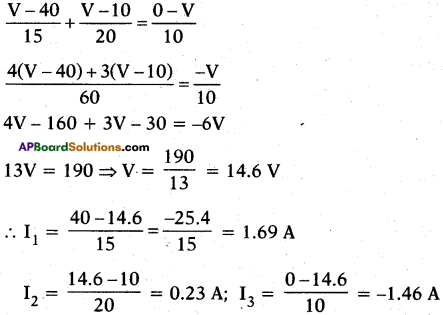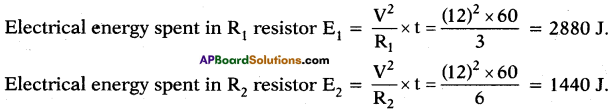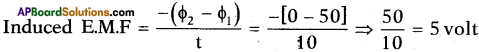AP State Board Syllabus AP SSC 10th Class Physical Science Important Questions Chapter 11 Electric Current.
AP State Syllabus SSC 10th Class Physics Important Questions 11th Lesson Electric Current
10th Class Physics 11th Lesson Electric Current 1 Mark Important Questions and Answers
Question 1.

Find the quantity of current in the above circuit. (AP March 2017)
Answer:
R = 3 + 5 + 2 = 10 Ω
I = \(\frac{1.5}{10}\) = 0.15 A.
Question 2.
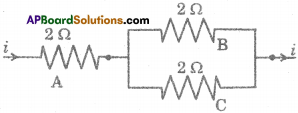
Three resistors A, B and C are connected as shown in the figure. Each of them dissipates energy to a maximum of 18 W. Find the maximum current that can flow through the three resistors. (TS March 2015)
Answer:
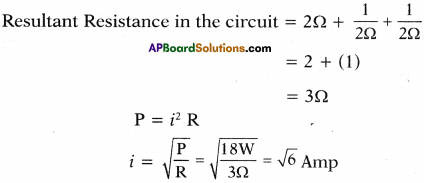
Question 3.
What happens if we use a fuse made up of same wire which is used to make the electric circuit? (TS March 2017)
Answer:
It doesn’t work as a fuse. If high voltage occurs fuse do not melt and circuit will not be opened / breaked. So home appliances will be damaged.

Question 4.
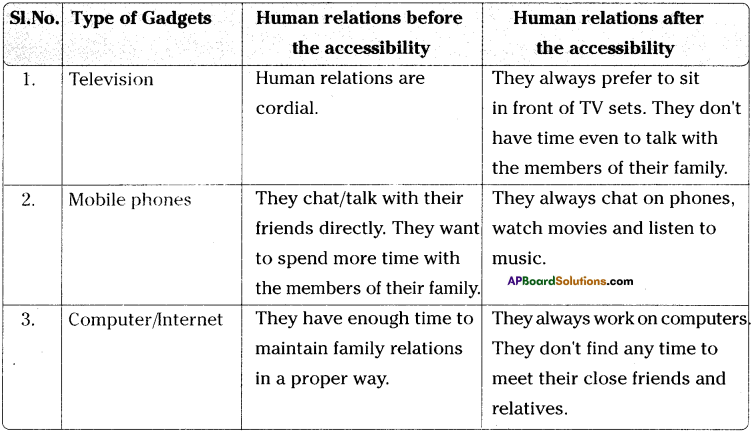
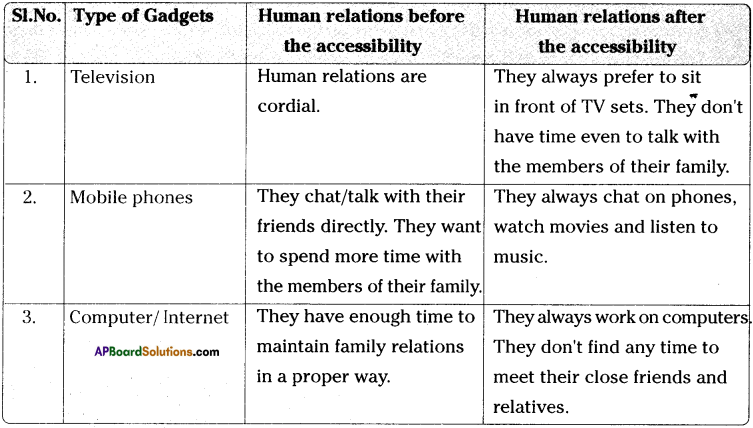
Write any two differences between ohmic and non- ohmic conductors. (TS June 2018)
Answer:
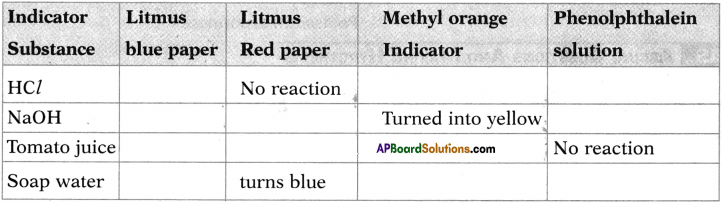
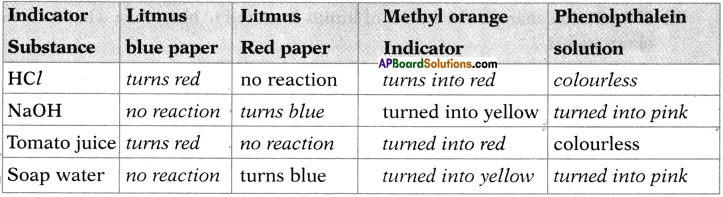
| Ohmic conductors |
Non-ohmic conductors |
| Ohmic conductors follow the Ohms law. |
Non-ohmic conductors do not follow the Ohms law. |
| Ohmic conductors are electric conductors. |
Non-ohmic conductors are semiconductors. |
| V-I graph of ohmic conductors is a straight line. |
V-I graph of non-ohmic conductors is a curve. |
Question 5.
Draw the electric circuit with the help of a Battery, Voltmeter, Ammeter, Resistance and connecting wires. (TS March 2018)
Answer:
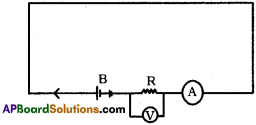
Question 6.
What happens, if the household electric appliances are connected in series? (TS March 2019)
Answer:
If all household appliances are connected in series, then if one appliances stop working due to failure then all the appliances stops working due incomplete circuit.
Question 7.
Define lightning.
Answer:
Lightning is an electric discharge between two clouds or between cloud and earth.
Question 8.
Define drift speed or drift velocity.
Answer:
The electrons in the conductor move with a constant average speed called drift speed or drift velocity.

Question 9.
Define conductors.
Answer:
The materials which can conduct electricity are called conductors.
Eg : Copper, Silver, Aluminium.
Question 10.
Define insulators.
Answer:
The materials which can’t conduct electricity are called insulators or non – conductors.
Eg: Wood, Rubber.
Question 11.
Define semi-conductors.
Answer:
The materials whose resistivity is 105 to 1010 times more than that of metals and 1015 to 1016 times less than that of insulators.
Eg: Silicon, Germanium,
Question 12.
How does a battery work?
Answer:
In a circuit, the battery stores chemical energy and this energy converts into electric energy. Thus a battery works.
Question 13.
Define lattice.
Answer:
Conductors like metals contain a large number of free electrons while the positive ions are fixed in their locations. The arrangement of the positive ions is called lattice.
Question 14.
Define potential difference.
Answer:
Work done by the electric force on unit positive charge to move it through a distance is called potential difference.

Question 15.
Define electromotive force.
Answer:
Electromotive force (emf) is defined as the work done by the chemical force to move unit positive charge from negative terminal to positive terminal of the battery.
Question 16.
Define resistance of a conductor.
Answer:
The obstruction to the motion of the electrons in a conductor is called resistance of a conductor.
Question 17.
Define resistivity (ρ).
Answer:
Resistivity is a constant.

Question 18.
Write Ohm’s formula.
Answer:
V = IR, where V is the potential difference (voltage), I is the electric current and R is the resistance.
Question 19.
Define resistor.
Answer:
The material which offers resistance to the motion of electrons is called a resistor.
Question 20.
What is a multimeter?
Answer:
It is an electronic measuring instrument that combines several measurement functions in one unit.
Question 21.
Define electric power.
Answer:
The product of voltage and electric current is called electric power.
Question 22.
Define electric energy.
Answer:
The product of electric power and time is called electric energy.
Question 23.
What is lattice?
Answer:
The arrangement of positive ions in a conductor is known as lattice.

Question 24.
Name two special characteristics of fuse wire.
Answer:
High resistivity and low melting point.
Question 25.
Name two special characteristics of heating coil.
Answer:
High resistivity and high melting point.
Question 26.
How does resistivity vary with material of conductor?
Answer:
The resistivity is less for a good conductor and is large for a bad conductor.
Question 27.
If length of a particular conductor increased by two times and its area of crosssection decreased by four times, then what happens to its resistivity?
Answer:
The resistivity of the conductor does not change because resistivity does not depend on dimensions of conductor.
Question 28.
What does the slope of V -1 graph for an Ohmic conductor represent?
Answer:
For an Ohmic conductor the slope of V -1 graph represents the resistance.
Question 29.
Express the units of ohm in terms of volt and ampere.
Answer:
1) The SI unit of resistance is ‘Ohm’.
2) Ohm = \(\frac{\text { Volt }}{\text { Ampere }}\)
Question 30.
What is the resultant resistance of this combination?
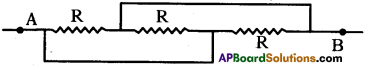
Answer:
R, R, R Ω resistances are in parallel.
⇒ Resultant resistance = \(\frac{R}{3}\)
Question 31.
What are the quantities are conserved in Kirchhoff’s and Is’ laws?
Answer:
According to Ist law, charge, and 2nd law, energy are conserved.
Question 32.
If the length and radius of a conductor are both halved. What happend resistance of wire?
Answer:
Length and radius are halved.
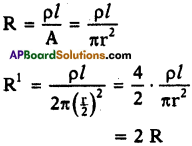
Resistance is doubled.
Question 33.
If work done is W and the charge that flows through is Q, then what is the equation 1 of potential difference?
Answer:

Question 34.
How many electrons constitutes a current of one ampere?
Answer:
6.25 × 1018 electrons in one second.
Question 35.
What are the maximum and minimum resistances are prepared by 30Ω, 30Ω, 30Ω?
Answer:
Maximum resistance = 30 + 30 + 30 = 90Ω
Minimum resistance = \(\frac{30}{3}\) = 10 Ω

Question 36.
Electric current I = nqA,vd. Write the representation of letters.
Answer:
n = Electron density
A = Area of cross – section
vd = drift velocity
q = charge of electron .
Question 37.
What is the resistance of bulb marked 60W and 120V?
Answer:
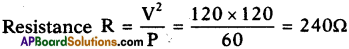
Question 38.
From figure, if VA = 10V, then VB = ?
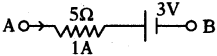
Answer:
VA – IR – E = VB
10 – 1 × 5 – 3 = VB
10 – 5 – 3 = VB
2 = VB
∴ VB = 2 volts
Question 39.
Write the examples of non-ohmic conductors.
Answer:
Non-ohmic conductors are electrolytes, semi conductors, vacuum tubes.
Question 40.
What is meant by electric shock?
Answer:
When the current flows through the body the functioning of organs inside the body gets disturbed. This disturbance inside the body is felt as electric shock.
Question 41.
There is no electric shock on bird, when stand on the Electric wires. Why?
Answer:
When the bird stands on a high voltage wire, there is no potential differences between the legs of the bird, so no current passes through the bird.
Question 42.
Which factors are influence the resistivity of wire?
Answer:
a) Temperature,
b) Nature of material.

Question 43.
The length of wire is doubled and area of cross-section also doubled. What is the change in resistivity.
Answer:
Resistivity is independent of length and Area of cross-section.
Resistivity is not change.
Question 44.
A battery of 6V is applied across a resistance of 15Ω. Find the current flowing through the circuit.
Answer:

Question 45.
The formula V = I R is applicable for what substances?
Answer:
V = IR is applicable for
a) Ohmic conductors,
b) Non-ohmic conductor.
Question 46.
Resistance of a conductor of length 75 cm is 3.250. Calculate the length of a similar conductor, whose resistance is 16.25Ω.
Answer:
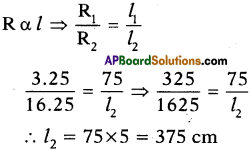
Question 47.
If four wires of each resistance R are joined to form a square, then what is the resistance between its opposite vertices?
Answer:
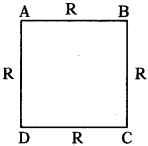
Resistance of ABC = 2R
Resistance of ADC = 2R
2R & 2R are parallel ⇒ Resultant = ‘R’
Question 48.
How much power consumption in a day of 100W television utilised 10 hours?
Answer:
Power consumption = \(\frac{100 \mathrm{~W} \times 10 \mathrm{~h}}{1000}\)
1 KWH or 1 unit.
Question 49.
How should we connect a voltmeter to measure voltage?
Answer:
The voltmeter must be connected in parallel to the electric device to measure the potential difference across the ends of the electric device.
Question 50.
How are ammeter and voltmeter connected in a circuit?
Answer:
Ammeter is always connected in series and voltmeter is always connected in parallel in a circuit.

Question 51.
Is the voltmeter connected in series or parallel in circuit? Why?
Answer:
Voltmeter should be connected parallel in the circuit to measure the potential difference between two points of conductor.
Question 52.
State whether the home appliances like Fridge, TV, Computer are connected in series or parallel Why?
Answer:
They are connected in parallel because if any one device is damaged, the rest will work as usual because the circuit does not break.
Question 53.
Why are copper wires used as connecting wires?
Answer:
Copper is a good conductor of electricity so copper wires are used as connecting wires.
Question 54.
Why is the fuse wire fitted in a porcelain casing?
Answer:
Porcelain is an insulator of electricity. So fuse wire is fitted in a porcelain casing.
Question 55.
Draw the diagram of potential – current of a copper conductor.
Answer:
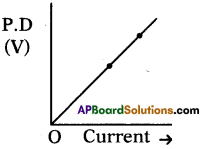
Temperature T = 27°C
It is a straight line passing through origin.
Question 56.
Draw the shape of V – I graph for a silicon.
Answer:
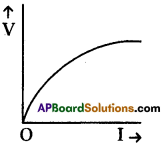
Silicon is a semi conductor.
It is not obey the Ohm’s law.
Question 57.
Is there any application of Ohm’s Law in daily life?
Answer:
- Electrical device like electric bulb, iron box and regulators are some applications of Ohm’s Law.
- Fuse in household circuits is also another application of Ohm’s Law.
Question 58.
Two wires of the same material and same length have radii r1 and r2 respectively. Compare (i) their resistance, (ii) their resistivities.
Answer:
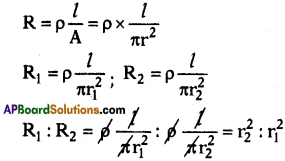
Resistivity for a same material is same. So their ratio =1:1.
Question 59.
A wire of resistance is doubled on itself, then what is its new resistance?
Answer:
Suppose length is l, area of cross-section is A and resistance is R.
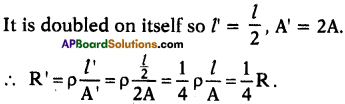
Question 60.
Calculate effective resistance between points A and B.
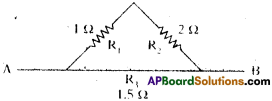
Answer:
R1 = 1 Ω, R2 = 2 Ω are in series. So R’ = 1 + 2 = 3 Ω.
Now R3 and R’ are in parallel.
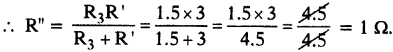
Question 61.
A fuse is rated 8A. Can it be used with an electrical appliance of rating 5 KW and 200 V?
Answer:
Given P = 5 KW = 5 × 1000 = 5000 W; V = 200 V.
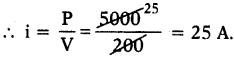
So a fuse of rate 8 A is not suitable because it uses current of 25 A.
Question 62.
A current of 2A is passed through a coil of resistance 75 Ω for 2 minutes. How much heat energy is produced?
Answer:
Given i = 2A, R = 75 Ω and t = 2 min. = 2 × 60 sec. = 120 sec.
Heat energy produced H = i² Rt = 2² × 75 × 120 = 36000 J = 36 KJ.
Question 63.
What is ,the resistance under normal working conditions of a 240 V electric lamp rated at 60 W?
Answer:
P = 60 W; V = 240V.
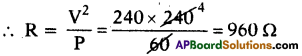
Question 64.
State the use of Ammeter. How should the Ammeter be connected in electric circuit?
Answer:
Ammeter should be connected in series in a circuit.
Question 65.
What is current value of x?

Answer:
2 + 3 = 5 of ‘A’
5 – 1 = 4 of ‘B’
2x = 4
x = 2 A at ‘C’
Question 66.
What is the value of VA, when VB = 8V?

Answer:
VA – 6 × 1 – 3 = VB
VA – 6 – 3 = 8
VA = 8 + 9
VA = 17 volts
Question 67.
In the figure, how much current passing through 6Ω resistor?
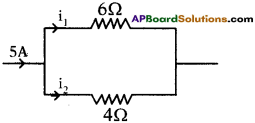
Answer:
Answer:
R1 : R2 = 6 : 4
R1 : R2 = 3 : 2
i1 : i2 = R2 : R1 = 2 : 3
∴ i1= 2A
i2 = 3A
10th Class Physics 11th Lesson Electric Current 2 Marks Important Questions and Answers
Question 1.
Observe the graph of potential difference (V) drawn between two ends of a conductor and current (I) passing through it. Answer the following questions :
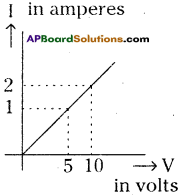
a) Which law is used to explain the graph? State it.
b) What is the resistance of the conductor? (AP June 2017)
Answer:
a) Ohm’s law is used to explain the given graph.
Ohm’s Law :
The potential difference between the ends of a conductor is directly proportional to the electric current passing through it at constant temperature.
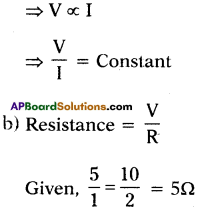
Question 2.
Draw the experimental set-up to verify that V/I is constant for a conductor. (TS March 2016)
Answer:
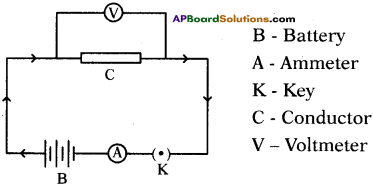
Question 3.
A house has 3 tube-lights of 20 watts each. On the average, all the tube-lights are kept on for five hours. Find the energy consumed in 30 days. (AP SCERT : 2019-20)
Answer:
Number of tube lights = 3
Wattage = 20 watts each
Consumed hours = 5 hrs
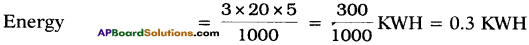
Consumed energy for 30 days = 0.3 × 30 = 9 KWH (or) 9 units.
Question 4.
How do the resistors 6 Ω, 10 Ω to be connected in a circuit to get minimum resistance? Find the resultant resistant of the circuit. (TS June 2019)
Answer:
- Resistors 6Ω, 10Ω should be connected in parallel connection in a circuit to get minimum resistance.
- Resultant resistance
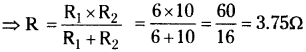
Question 5.
Write two examples for ohmiq and non-ohmic materials each. (TS June 2019)
Answer:
Ohmic materials : Copper, Aluminium
Non – ohmic materials : Germanium, Silicon
Question 6.
Give reasons for using lead in making fuses.
Answer:
- Lead is used in making fuses because it has low melting point EK resistivity.
- If the current in the lead wire exceeds certain value, the wire will heat up and melt, so the circuit in the households is opened and all the electric devices are saved.
Question 7.
How can we decide the direction of electric current in a conductor?
Answer:
We know I = nqAvd. In this n and A are positive. Hence the direction of current is determined by the signs of the charge ‘q’ and drift speed vd.
- For electrons, q is negative and vd is positive. Then the product of q and vd is negative. So the direction of electric current is opposite to the flow of negative charge.
- For positive charge, the product of q and vd is positive. Hence, the direction of electric current can be taken as the direction of flow of positive charges.

Question 8.
What are the devices used in a circuit?
Answer:
1) Ammeter :
It is used to measure current.
2) Volt meter:
It is used to measure potential difference across the ends of conductor.
3) Rheostat:
It varies current in a circuit.
4) Switch :
It is useful to make a circuit or break a circuit.
5) Cell:
It is source of electric energy in the circuit.
6) Multimeter :
It is useful to measure current, voltage and resistance in the circuit.
Question 9.
A student says “Potential difference and Emf are same.” Justify your answer.
Answer:
Both are different because potential difference is the work done by the electric force on unit positive charge to move it through a distance between two points whereas emf is the chemical force to move unit positive charge from negative terminal to positive terminal of the battery.

Question 10.
Define Ohmic and non-ohmic conductors and give two examples each of them.
Answer:
Ohmic conductors :
The conductors which obey Ohm’s law are called ohmic conductors, e.g, : Copper, Iron.
Non-ohmic conductors :
The conductors which do not obey Ohm’s law are called non-ohmic conductors, e.g. : Semiconductors, Electrolytes.
Question 11.
Explain the Junction law of Kirchhoff.
Answer:
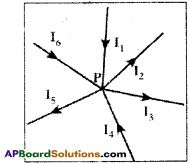
- At any junction point the sum of the currents into the junction must be equal to the sum of currents leaving the junction.
- There is no accumulation of electric charges at any junction in a circuit.
I1 + I4 + I6 = I5 + I2 + I3
Question 12.
Write differences between overloading and short circuiting.
Answer:
Current chooses a path which has least resistance. So sometimes electrical appliances get damaged by passage of excess of current due to short circuit.
When so many electrical appliances are connected to the same electrical main point, maximum current can be drawn from the mains which causes overheating and may cause a fire which is called overloading.
Question 13.
The V-I graph for a series combination and for parallel combination of two resistors is shown in figure. Which of the two A or B, represent parallel combination? Give reason for your answer.
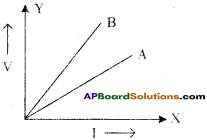
Answer:
i) For same change in I, change in V is less for the straight line A than for the straight line B (i.e., straight line A is less steeper than B).
ii) So the straight line A represents small resistance, while straight line B represents more resistance.
iii) In parallel combination, the resistance decreases, while in series combination, the resistance increases, so A represents a parallel combination.
Question 14.
Two resistors are joined with a battery such that
a) same current flows in each resistor.
b) potential difference is small across each resistor.
c) equivalent resistance is less than either of the two resistors.
d) equivalent resistance is more than either of the resistors.
State how are the resistors connected in each of the above case.
Answer:
a) Since same current is flowing in each resistor, they are connected in series.
b) Potential difference is same across same resistor that shows they are connected in parallel.
c) Equivalent resistance is less than the either of the two resistances which shows they are connected in parallel because in parallel connection resistance decreases.
d) Equivalent resistance is more than the individual resistances which indicate that they are connected in series. Since in series connection resistance increases.

Question 15.
Which of the cables, one rated 5A and other 15 A will be thicker wire? Give reason for your answer.
Answer:
i) To carry larger current, the resistance of wire should be low, so its area of cross-section should be large.
ii) Because the second cable carrying 15 A ampere current which indicates it has low resistance that is more surface area which implies it is thicker wire.
Question 16.
When a potential difference 30V is applied across a resistor, it draws a current of 3A. If 20V is applied across the same resistor, what will be the current?
Answer:
Situation : 1
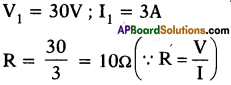
Situation : 2

Question 17.
Is Ohm’s Law universally applicable for all conducting elements? If not, give example of elements which do not obey the Ohm’s Law.
Answer:
- Ohm’s Law is not applicable for all conducting elements.
- For example, some semi-conductors like silicon, germanium do not obey the Ohm’s Law.
- Those which do not obey Ohm’s Law are called non-ohmic materials.
- LED’s are non-ohmic materials.
Question 18.
Alloys are used in electrical heating devices rather than the pure metal. Why?
Answer:
Alloys are homogeneous mixtures of two or more metals. Generally alloys have more resistivity than the metals from which they have been prepared. As the resistivity increases heating effect of conductor also increases. So alloys are preferred in heating devices.
Question 19.
A switch should not be touched with wet hands. Why?
Answer:
- A switch should not be touched with wet hands.
- If water reaches the live wire, it forms a conducting layer between the hand and the live wire of the switch through which the current passes to the hand, and the person may get a total shock.
Question 20.
Which material is used for power transmission? Why?
Answer:
- The wires which are used for connections and for power transmission are made of material such as copper or aluminium.
- The reason is their resistivity is very small and they are made thick so that their resistance can be considered to be negligible.
- Further, the loss of electrical energy due to heating is also negligible in them.

Question 21.
Which material is preferred for heating element? Why?
Answer:
- The heating elements or resistance wires (or standard resistors) are made of material such as nichrome, manganin, constantan, etc. for which the resistivity is quite large and the effect of change in temperature on their resistance is negligible.
- So electrical energy is converted into heat energy when current passes through the wire.
Question 22.
Draw the symbols of the following.
i) Battery
ii) Resistance
iii) Ammeter
iv) Voltmeter
v) Key
vi) Rheostat
Answer:
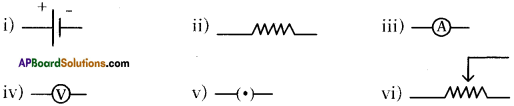
Question 23.
Draw V-I graphs of Ohmic and non-ohmic conductors.
Answer:
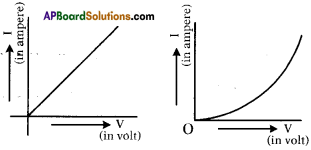
Question 24.
Draw a circuit diagram with a cell, an electric bulb, an ammeter and plug key.
Answer:
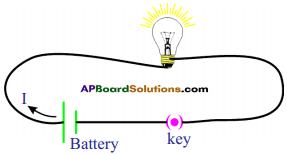
Question 25.
Draw a circuit diagram to verify the Ohm’s Law.
Answer:
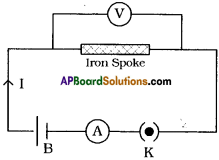
Question 26.
If 60 C of charge passes through a conductor for 1 minute, find the current through the conductor.
Answer:
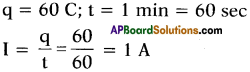
Question 27.
The work done in moving 6 C of charge through a circuit is 12 J. Find the potential difference in the circuit.
Answer:
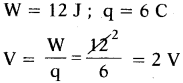
Question 28.
Resistance of two resistors are 6 Ω, 12 Ω respectively. Find the resultant resistance if the resistors are connected (1) in series (2) in parallel.
Answer:
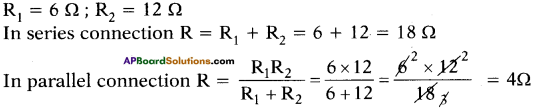
Question 29.
10 equal resistors of resistance 20 are connected in a circuit. Find the resultant resistance if they are connected in series or in parallel ?
n = 10; R = 20 Ω
In series connection resultant resistance R’ = nR = 10 × 20 = 200 Ω

Question 30.
From the figure find the current through 6 Ω, 12 Ω resistors and find the resultant resistance in the circuit and also find current in the circuit.
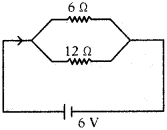
Answer:
R1 =6 Ω; R2 = 12 Ω; V = 6 V
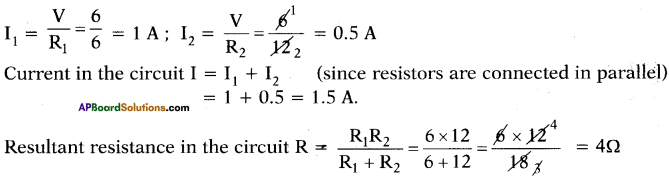
Question 31.
Find which has greater resistance. 1 KW heater or a 100 W tungsten bulb, both marked for 230 V.
Answer:
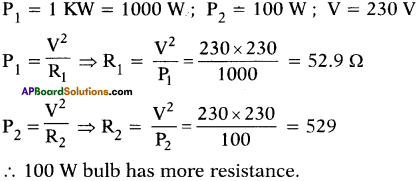
Question 32.
Two wires (one is copper and other is aluminium) have equal area of cross-section and have the same resistance. Find which one is longer.
Answer:
Suppose the resistance of copper and aluminium wires are R1 and R2. Suppose their area of cross-section is A.
The resistivity of copper (ρ1) = 1.68 × 10-8
The resistivity of aluminium (ρ2) = 2.82 × 10-8
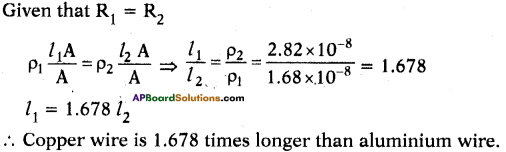
Question 33.
Three equal resistances are connected In series, then in parallel. What will be the ratio of their resultant resistances?
Answer:
Suppose the resistance of equal resistors is ‘R’. Suppose they are connected in series. Then their equivalent resistance R’ = R + R + R = 3R
If they are connected in parallel their equivalent resistance R” = \(\frac{R}{3}\)
∴ Ratio of resultant resistances = R’: R” = 3R : \(\frac{R}{3}\) = 9 : 1
Question 34.
How is Ideal earthing helpful during short circuiting?
Answer:
- During short circuiting an excessive current flows through the live wires.
- It will pass to earth through the earth wire if there is local earthing otherwise it may cause a fire due to overheating of the live wires.

Question 35.
You have three resistor values 2Ω, 3Ω and 5Ω. How will you join them Sd that the total resistance Is less than 2Ω? Find resultant resistance.
Answer:
Given R1 = 2Ω, R2 = 3Ω, R3 = 5Ω.
The resistors should be joined in parallel.
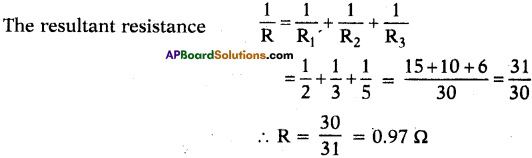
Question 36.
An electric kettle Is rated 3 KW, 250 V. Give reason whether this kettle can be used in a circuit which contains a 13 A fuse?
Answer:
V = 250 V, P = 3KW = 3000 W
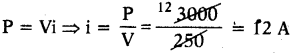
The fuse is suitable because safe limit of current for kettle is 12 A.
Question 37.
Two resistors of 4 Ω and 6 Ω are connected parallel. The combination is connected across 6 V battery of negligible resistance. Find, i) the power supplied by the battery and ii) the power dissipated in each resistance.
Answer:
i) R1 = 4 Ω, R2 = 6 Ω, V = 6V.
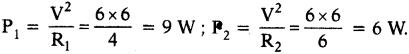
ii) In parallel connection the resultant resistance
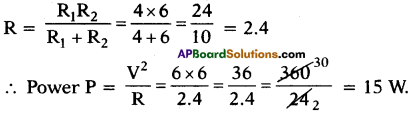
Question 38.
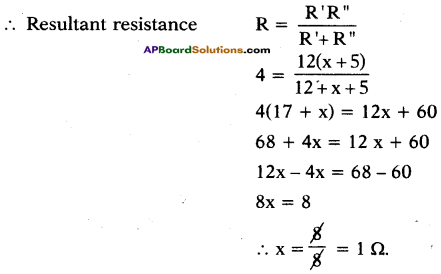
In the circuit shown below calculate the value of x if the equivalent resistance between A and B is 4 Ω.
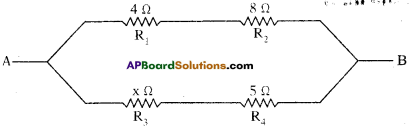
Answer:
Given R1 = 4 Ω, R2 = 8Ω, R3 = x Ω and R4 = 5 Ω.
And resultant resistance R = 4 Ω.
R1 and R2 are in series.
Resultant resistance R’ = R1 + R2 = 4Ω + 8Ω = 12 Ω.
R3 and R4 are also in series.
Resultant resistance R” = R3 + R4 = x + 5
R’ and R” are in parallel.
Question 39.
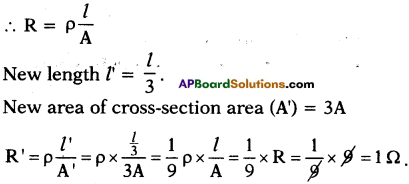
A wire of 9 Ω resistance having 30 cm length is tripled on itself. What is its new resistance?
Answer:
Given R = 9 Ω, l = 30 cm and suppose area of cross-section A.
10th Class Physics 11th Lesson Electric Current 4 Marks Important Questions and Answers
Question 1.
A house has four tube-lights, three fans and a television. Each tube-light draws 40 W. The fan draws 80 W and the television draws 100 W. On an average, all the tube-lights are kept on for 5 hours, all fans for 12 hours and the television for 6 hours everyday. Find the cost of electric energy used in 30 days at the rate of Rs. 3.00 per KWH. (AP March 2015)
Answer:
The power used by
1) Four tube-lights of 40 W for 5 hours in 30 days
= 4 × 40 × 5 × 30 = 2400 WH
2) Three fans of 80 W for 12 hours in 30 days
= 3 × 80 × 12 × 30 = 86400 WH
3) One television of 100 W for 6 hours in 30 days
= 1 × 100 × 6 × 30 = 18000 WH
4) Total electric energy used
= 24000 + 86400 + 18000 = 128400 WH
W.H. converted into K.W.H. = 128.4 K.W.H. \(\left[\because \frac{128400}{1000}\right]\)
Cost of 1 unit = Rs. 3.00
Amount to be paid for 128.4 units = 128.4 × 3 = Rs. 385.20
Question 2.
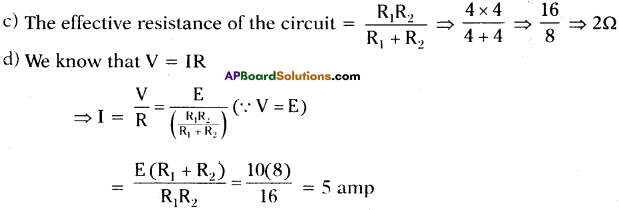
Observe the given circuit. (AP June 2017)
R1 and R2 are two resistors and R1 = R2 = 4Ω. Emf of the battery E is 10 V.
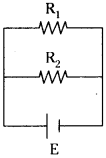
Answer the following questions.
a) How are the resistors R1 and R2 connected in the circuit ?
b) What is the potential difference across R1?
c) What is the effective resistance of the circuit?
d) What is the total current drawn from the battery?
Answer:
a) Resistors R1 and R2 are connected in parallel in the given circuit.
b) The potential difference across R1 is ‘E’ volts = 10 V.
Question 3.
State Kirchhoff’s Loop law and explain. (AP June 2018)
Answer:
Loop law :
The algebraic sum of the increases and decreases in potential difference across various components of the circuit in a closed circuit loop must be zero.
Explanation :
Let us imagine in a circuit loop the potential difference between the two points at the beginning of the loop has a certain value. As we move around the circuit loop and measures the potential difference across each component in the loop, the potential difference may decrease or increase depending upon the nature of the element like a resistor or battery. But when we have completely traversed the circuit loop and arrive back at starting point. The net change in the potential difference must be zero. Thus the algebraic sum of changes in potential difference is to be zero.
(OR)
Let us apply loop law to a circuit as below.
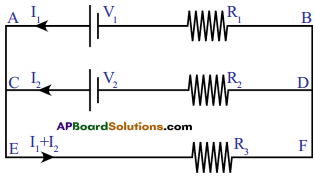
for the loop ACDBA
– V2 + I2R22 – I1R1 + V1 = 0
for the loop EFDCE
-(I1 + I2)R3 – I2R2 + V2 = 0
for the loop EFBAE
-(I1 + I2)R3 – I1R1 + V1 = 0
Question 4.
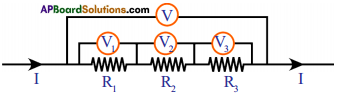
Observe the above diagram and answer the following. (AP March 2018)
a) Are all the resistors connected in parallel or series?
b) What is the equivalent resistance of the combination of three resistors?
c) In this system, which physical quantity is constant?
d) If R1 = 2Ω, R2 = 3Ω, R3 = 4Ω. find equivalent resistance.
Answer:
a) Connected in series.
b) Req = R1 + R2 + R3
c) Current (I)
d) Req = R1 + R2 + R3
=2 + 3 + 4
= 9Ω
Question 5.
How do you verify that resistance of a conductor of uniform cross-section area is proportional to the length of the conductor at constant temperature? (TS March 2015)
Answer:
Aim :
To verify the relation between re.M+iance and lenath of the conductor.
1) Required material :
Wires or spokes of different lengths with same cross-section area of the same metal.
2) Battery
3) Ammeter
4) Key
5) Connecting wires.
Procedure :
1) Construct a circuit with Battery, Ammeter, Switch (key) and connecting wires, keeping some space at the both ends.
2) Connect the selected wires or spokes at the ends to complete the circuit.
3) Connect the wires or spokes individually and record the current using ammeter.
Conclusion :
If the current flowing in the circuit decreases with an increase in the length of the wire or spokes (Resistance increases), we can say that the resistance of the conductor is proportional to the length of the conductor.
Question 6.
What are the factors affecting the resistance of an electric conductor? Explain any two factors. (TS June 2015)
Answer:
The factors affecting the resistance of an electrical conductor are
- Nature of material
- Temperature
- Length of the conductor
- Area of cross-section of conductor
Explanation :
- As the temperature increases the resistance increases and vice versa.
- As the material changes resistance changes.
- Resistance is directly proportional to length of the conductor (if T and A are kept constant). R ∝ l
- Resistance is inversely proportional to area of cross-section (if 1 and T are kept constant). R ∝ \(\frac{1}{A}\)

Question 7.
What is the relationship between length of a conductor and its resistance? Write the experimental procedure to verify that relationship. (TS Junc 2017)
Answer:
- The resistance of a conductor is directly proportional to its lenght for a constant potential difference.
- Take iron spokes of different lengths with same cross-sectional arreas.
- Make a circuit by connecting an iron spoke with battery, ammeter and switch in series.
- Put the switch on and allow the current to pass in the circuit. Measure the ammeter reading.
- Repeat this for other lengths of the iron spokes. Note the corresponding values of currents.
- The resistance of each spoke increases with increase in the length of the spoke.
Question 8.
Find the resultant resistance for the following given arrangement. Find the current when this arrangement is connected with 9 V battery. (TS March 2017)

Answer:
The diagram is not clear so award 4 marks in the public examinations.
Question 9.
12 V battery is conncected in a circuit and to this 4Ω, 12Ω resistors are connected in parallel, 3Ω resistor is connected in series to this arrangement. Draw the electric circuit from this information and find the current in the circuit. (TS June 2018)
Answer:
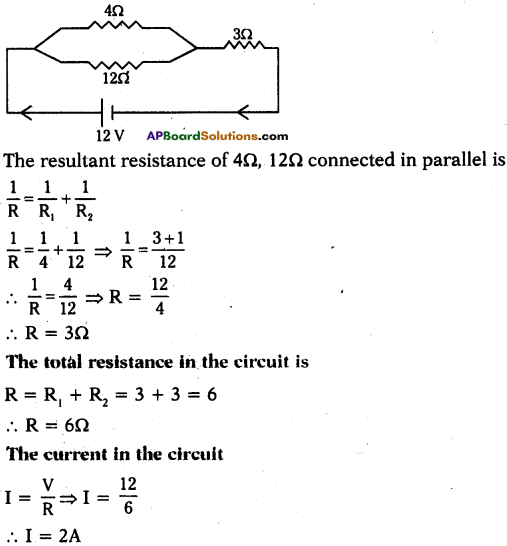
Question 10.
In a circuit,60V battery, three resistances R1 = 10 Ω, R2 = 20 Ω and R3 = x Ω are connected in series. If 1 ampere current flows in the circuit, find the resistance in R3 by using Kirchhoffs loop law. (TS March 2018)
Answer:
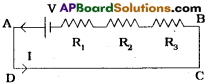
Given
I = 1 amp
R1 = 10Ω
R2 = 20Ω
R3 = xΩ
V = 60V
In ADCBA loop
– IR3 – IR2 – IR1 + V = 0
– lx – 1 × 20- 1 × 10 + 60 = 0
– x – 30 + 60 = 0
– x + 30 = 0
x = 30Ω
Question 11.
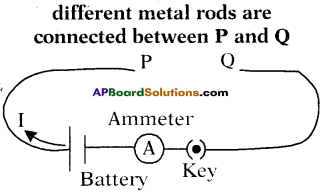
List out the material required for the experiment “The effect of increasing of cross-section of a conductor upon its resistance” and write the experimental procedure. (TS June 2019)
Answer:
Aim :
To show that the effect of increasing of cross-section of a conductor upon its resistance.
Required Material :
Battery eliminator, Key, Ammeter, Manganin wires of equal lengths but different cross sectional areas, Copper connecting wires.
Procedure :
- Collect manganin wires of equal lengths but different cross sectional areas.
- Make a circuit as shown in the figure.
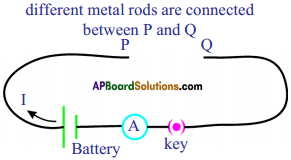
- Connect one of the wires between points ‘P’ and ‘Q’.
- Note the value of the current using the ammeter connected to the circuit and note it.
- Repeat this with other wires.
- Note the corresponding values of currents in each case and note them.
Conclusion :
We can notice that the current flowing through the wire increases with increasing their cross sectional area.
Question 12.
Derive an expression to find drift velocity of electrons.
Answer:
- Consider a conductor with cross sectional area A. Assume that the two ends of the conductor are connected to a battery to make the current flow through it.
- Let ‘vd‘ be the drift speed of the charges and ‘n’ be the number of charges present in the conductor in a unit volume.
- The distance covered by each charge in one second is ‘vd‘
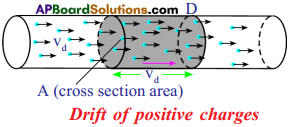
- Then the volume of the conductor for this distance = Avd
- ∴ The number of charges contained in that volume = n.Avd
- Let q be the charge of each carrier.
- Then the total charge crossing the cross sectional area at position D in one second is ‘n q Ad‘.
This is equal to electric current.
∴ Electric current I = n q Avd.
∴ vd = \(\frac{I}{nqA}\)
Question 13.
Derive an expression to measure emf of a battery.
Answer:
Electromotive force (emf) is defined as the work done by the chemical force to move unit positive charge from negative terminal to positive terminal of the battery.
1. Let this chemical force be Fc.
2. This chemical force does some work to move a negative charge ‘q’ from positive terminal to negative terminal against the electric force Fe. Let this work be ‘W’.
3. ∴ The work done by the chemical force to move 1 coloumb of charge from the
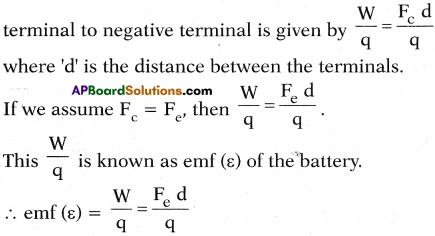
This S.I unit of emf is ‘volt’ and is measured using voltmeter.
Question 14.
What are the factors on which the resistance of conductor depends? Give the corresponding relation.
Answer:
- The value of resistance of a conductor depends on temperature for constant potential difference.
- Resistance of a conductor depends on the material of the conductor.
- Resistance of a conductor is directly proportional to its length, i.e., R ∝ l.
- Resistance of a conductor is inversely proportional to the area of cross-section of the material, i.e., R ∝ \(\frac{1}{A}\)

Question 15.
What do you mean by (i) short circuit (ii) overloading? What are the safety precautions taken to avoid these problems in domestic electric circuits?
Answer:
Short circuit:
Sometimes current chooses a path which has least resistance which is called short circuit.
Overloading :
The over heating due to drawing excess of current from a single main is called overloading.
Precautions to avoid damage due to short circuit and overloading.
- Using fuse
- Connecting electrical appliances in various mains.
- Earthing
Question 16.
A circuit is set up as shown in the figure. Calculate the current and potential difference across R1, R2 and R3, when
a) keys K1 and K2 are both closed,
b) key K1 is closed and K2 is open,
c) key K1 is open and K2 is closed.
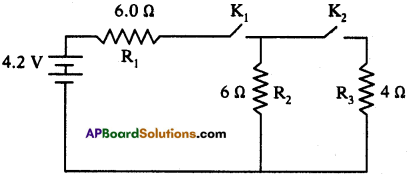
Answer:
a) When both the keys K1 and K2 are closed :
The resistors R1 and R3 are parallel.
So resultant resistance Rp = \(\frac{R_{1} R_{3}}{R_{1}+R_{3}}=\frac{6 \times 4}{6+4}\) = 2.4 Ω.
The resistor R1 and Rp are in series as shown in figure.
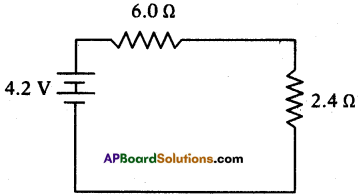
Total resistance of circuit Rs= R1 + Rp = 6 + 2.4 = 8.4 Ω
Current I = \(\frac{V}{R_{S}}=\frac{4.2}{8.4}\) = 0.5 =A.
Potential difference across R1 is V1 = IR1 = 0.5 × 6 = 3V.
Potential difference across the combination of R2 and R3 is
V’ = V – V1 = 4.2 – 3 = 1.2 V.
Now since R2 and R3 are in parallel,
Potential difference across R2 = Potential difference atross R3 = V’ = 1.2 V
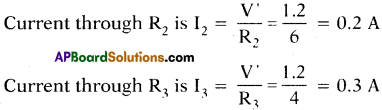
b) When the key K1 is closed and key K2 is open : The resistor R3 will not be in circuit.
The resistors R1 and R2 are in series.
Total, resistance Rs = R1 + R2 = 6 + 6=12 Ω.
Current I = \(\frac{V}{R_{s}}=\frac{4.2}{12}\) = 0.35 A
The same current will flow through each resistor R1 and R2.
Potential difference across R1 is V1 = IR1 = 0.35 × 6 = 2.1 V.
Potential difference across R2 is V2 = IR2 = 0.35 × 6 = 2.1 V.
The current and potential difference across R3 will be zero.
c) When key K1 is open and key K2 is closed :
No current flows through R1 R2 and R3 since the circuit is incomplete. Hence potential difference across R1, R2 and R3 is zero.
Question 17.
For the combination of resistance shown in figure, find the equivalent resistance between (a) C and D, (b) A and B.
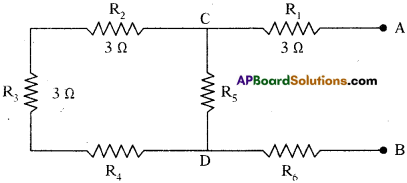
Answer:
a) Between C and D :
The resistors R2, R3 and R4 are in series. They can be replaced by an equivalent resistance Rs where
Rs = R2 + R3 + R4 = 3 + 3 + 3 = 9 Ω
The resistance R5 and Rs are in parallel between the points C and D.
The equivalent resistance between C and D then
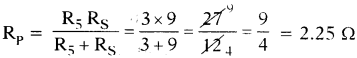
Thus the equivalent resistance between C and D is 2.25 Ω.
b) Between A and B :
Now the resistors R1, Rp and R6 are in series between the points a and B.
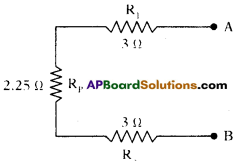
The equivalent resistance between A and B is
RAB = R1 + Rp + R6 = 3 +2.25 + 3 = 8.25 Ω.
Question 18.
How does resistance and resistivity vary with temperature?
Answer:
- For a metallic conductor, the resistance increases w ith the increase in temperature. The resistance of filament of bulb is more w hen it is glowing than when it is not glowing. The specific resistance or resistivity also increases with increase in temperature.
- For alloys (such as constantan and manganin), the resistance and the resistivity remains practically unchanged with the increase in temperature.
- For semi conductors (such as silicon, germanium, etc.), the resistance decreases with the increase in temperature.
eg.: the resistance of carbon also decreases with the increase in its temperature.

Question 19.
Why is a copper wire unsuitable for making fuse wire?
Answer:
- A fuse is a short piece of wire made up of a material of high resistivity and of low melting point so that it may easily melt due to overheating when current in excess to the prescribed limit passes through it.
- The thickness of wire is different in different fuses depending on the amount of current which is permitted to flow through them.
- Generally an alloy of lead and tin is used as the material of the fuse wire because it has a high resistivity and low’ melting point.
- A copper wire is unsuitable for using as fuse wire because copper has low resistivity and high melting point.
- Therefore the use of an ordinary’ wire as a fuse must be avoided.
Question 20.
Why is current rating of a fuse required?
Answer:
1) The electric wiring for light and fan circuit uses a thin fuse wire of low current carrying capacity because the line wire has a current carrying capacity of 5A.
2) Thicker fuse wires of higher current carry ing capacity (15 A) are used for large current consuming appliances such as air conditioner, geyser, washing machine, etc. because the line wire for such dev ices have current carrying capacity of 15A.
The current rating of a fuse in a circuit can be obtained by the following relation.

Question 21.
Describe the activity with the help of diagram to establish the relationship between Current (I) flowing in a conductor and potential difference (V) maintained across its ends.
Answer:
Aim :
To establish the relationship between Current (I) flowing in a conductor and potential difference (V) maintained across its ends.
Material required :
5 dry cells of 1.5 V each, conducting wires, an ammeter, a volt meter, thin iron spoke of length 10 cm, LED and key.
Diagram :
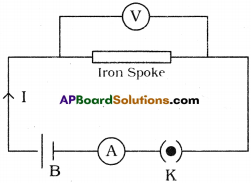
Procedure :
- Connect a circuit as shown in the figure.
- Solder the conducting wires to the ends of the iron spoke.
- Close the key.
- Note the readings- of current from ammeter and potential difference from volt meter in the given table,
- Now connect two cells (in series) instead of one cell in the circuit.
- Note the respective readings of the ammeter and voltmeter and record the values in the table.
- Repeat the same for three cells, four cells and five cells respectively.
- Record the values of V and I corresponding to each case in the table.
- Find \(\frac{V}{I}\) for each set of values.
Conclusion : The ratio of \(\frac{V}{I}\) is constant.
- From this activity we can conclude that the potential difference between the ends of the iron spoke (conductor) is directly proportional to the current passing through it. This means V ∝ I.
Question 22.
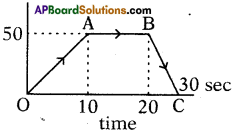
In an experiment to verify Ohm’s Law the following values are given below. Draw a graph of ‘I’ versus ‘V’. Show that the graph conforms Ohm’s Law and find the resistance of the resistor.
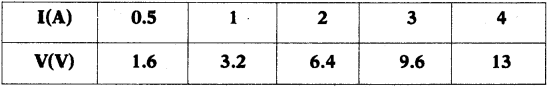
Answer:
1) Graph between ‘V’ and ‘I’.
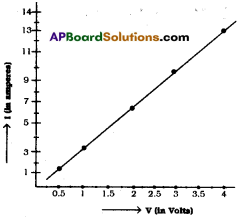
2) From the above graph,
Straight line §hows that the relation between potential difference (V) and current (I) as \(\frac{V}{I}\) is constant.
3) V ∝ I.
4) The potential difference between the ends of a conductor is directly prbportional to the electric current passing through it at constant temperature.
5) This is the Ohm’s Law and the graph conforms it.
Resistance of the resistor
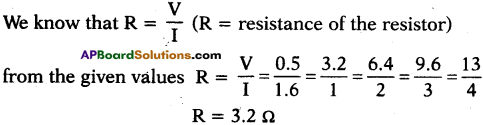
Question 23.
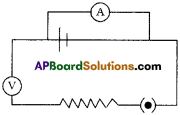
Identify the defects 1ft the circuit. Redraw it.
Answer:
Defects in the circuit:
- Ammeter was connected in parallel in the circuit.
- Volt meter was connected in series in the circuit.
- Positions of resistor and battery were reversed.
Correct diagram :

Question 24.
What is the advantage of MCB over fuse?
Answer:
- These days instead of fuses, Miniature Circuit Breakers (MCB) are used for each lighting circuit.
- They switch off the circuit in a very short time duration in case of short-circuiting or some fault in the line.
- After repairing the fault in the circuit, the MCB is again switched on.
- Thus, the use of MCB is better than a fuse. It avoids the inconvenience of connecting a new fuse wire and it is much safer due to its quick response.
Question 25.
A circuit is shown in the picture.
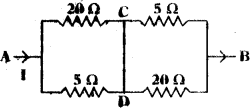
The current passing through A is I.
a) What is the potential difference between A and B?
b) What is the equivalent resistance between A and B?
c) What amount of current is flowed through C and D?
Answer:
a) According to KirchhofPs loop law the algebraic sum of increase and decrease in potential difference across various components of the circuit in a closed circuit loop must be zero.
So the potential difference across CD is zero because it is a closed loop.
b) Here 20 Ω, 5 Ω are parallel to each other and resultants are in series to each other. Resultant resistance of 20 Ω and 5 Ω.
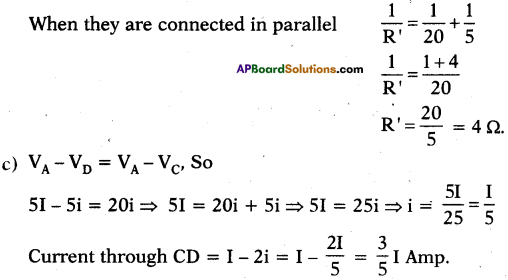
Question 26.
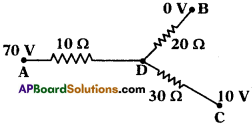
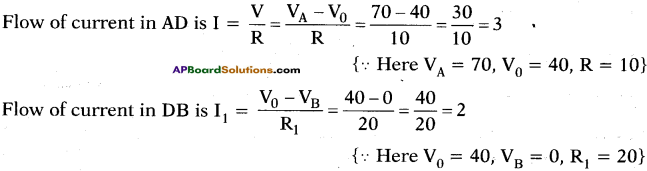
Observe the picture. The potential values at A, B, C are 70 V, 0 V, 10 V
a) What is the potential at D?
b) Find the ratio of the flow of current in AD, DB, DC.
Answer:
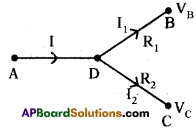
a) By following Ohm’s law potential difference is (V) = IR
In the given circuit we are applying junction laws.
‘D’ works as junction so, I = I1 + I2
Let p.d at D is V0.

b)
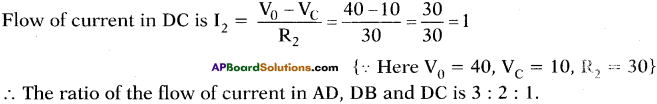
Question 27.
Observe the circuit R1 = R2 = R3 = 200 Ω. If reading of voltmeter is 100 V, resistance of voltmeter is 1000 Ω.
Find the Emf of the battery.
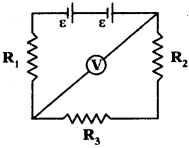
Answer:
Given values are R1 = R2 = R3 = 200 Ω.
and Voltmeter reading = V = 100 V.
Resistance of Voltmeter = Rv = 1000 Ω.
In the given circuit R1 and R2 are in series R = R1 + R2 = 200 + 200 = 400 Ω
Resultant resistance (400 Ω) and voltmeter (1000 Ω) are always in parallel.
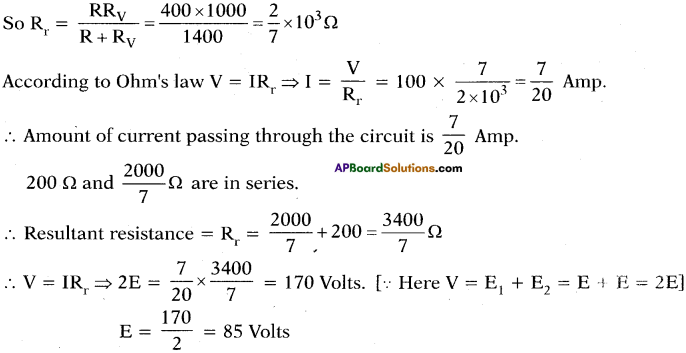
Question 28.
A circuit is made with a copper wire as shown in the diagram. We know that conductor’s resistance is directly proportional to its length. Calculate the equivalent resistance between points 1 and 2.
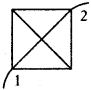
Answer:
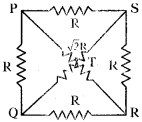
Let the resistance of the wire be ‘R’ and length of the wire be ‘l’.
The shape of the circuit be square length of the side (l) = R
In a square, diagonal is \(\sqrt{2}\) times its length = \(\sqrt{2}\)l
Resistance towards diagonal is \(\sqrt{2}\)R
The circuit diagrams for the given arrangement are along PTR and QTS is ineffective as no current flows through it.
PQ and PS are in series so effective resistance is R1 + R2 = R + R = 2R.
QR and SR are in series so effective resistance is R1 + R2 = R + R = 2R.
Redrawn of the circuit again as resultant resistance between the points 1 and 2 is
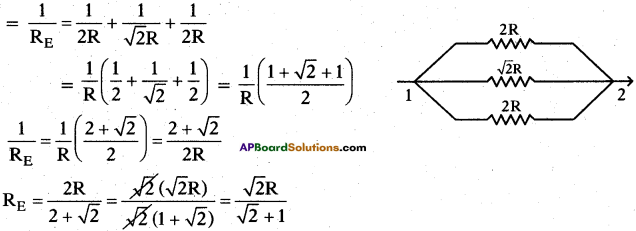
Question 29.
From the adjacent figure,
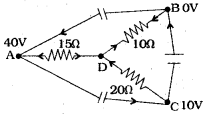
i) Find the potential at D.
ii) Find the current that passes through AD, DB and DC.
Answer:
Suppose from A and C current is flowing through the circuit and at B current is flowing away from the circuit. Suppose potential at D is V.
∴ I1 + I2 = I3 (where I1 I2 are current into the junction. I3 is the current away from the junction)
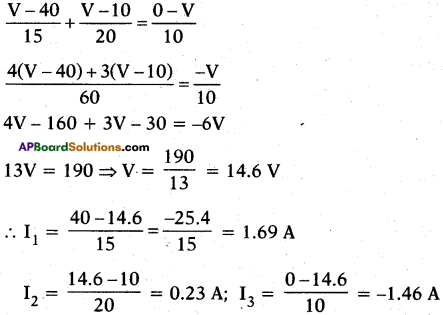
Question 30.
Find the electric current drawn from the battery of emf 8V from the given circuit.
Answer:
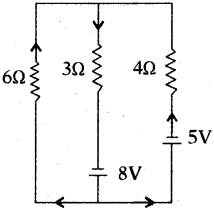
According Kirchhoffs loop law
6I1 + 3I1 – 8 = 0
9I1 = 8
I1 \(\frac{8}{9}\) = 0.89 A
∴ Cuttent in 8 V is 0.89 A
Question 31.
A household uses the following electric appliances.
i) Refrigerator of rating 400 W each for ten hours each day.
ii) Two electric fans of rating 80 W each for 12 hours each day.
iii) Six electric tubes of rating 18 W each for 6 hours each day. Calculate the electric bill of the household in a month if the cost per unit electric energy is Rs. 3.00.
Answer:
i) Electrical energy consumed by Refrigerator in a month (in KWH)
ii) Electrical energy consumed by two electrical fans in a month (in KWH)
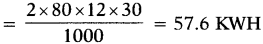
iii) Electrical energy consumed by electric tubes in a month (in KWH)
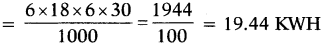
Total electrical energy consumed by all the electrical appliances
= 120 + 57.6 + 19.44 = 197.04 units
Cost of 1 unit = ₹ 3
Cost of 197.04 units = 197.04 × 3 = ₹ 591.12
Question 32.
What is the reason for connecting the fuse in the live wire?
Answer:
- The fuse is always connected in the live wire of the circuit.
- If the fuse is put in the neutral wire, due to excessive flow of current the fuse burns, current stops flowing in the circuit.
- But the appliance remains connected to the high potential point of the supply through the live wire.
- Now if a person touches the appliance, he may get a shock as the person will come in contact with the live wire through the appliance.
Question 33.
In the diagram given below, two resistors R1 and R2 of 3Ω and 6Ω respectively are connected in parallel across a battery of potential difference 12 V. Calculate the electrical energy consumed in 1 minute in each resistance.
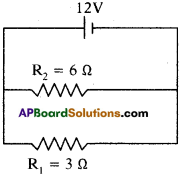
Answer:
Given R1 = 3 Ω, R2 = 6 Ω, V= 12 V, t = 1 min 60 sec.
The resistors R1 and R2 are connected in parallel, so the voltage V across each resistor is equal to 12 V.
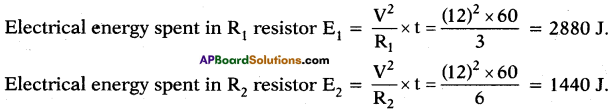
Question 34.
Calculate the electrical energy consumed in a month, in a house using 2 bulbs of 100 W each and 2 fans of 60 W each, if the bulbs and fans are used for an average of 10 hours each day.
If the cost per unit is ₹ 3, calculate the amount of electrical bill to be paid per month.
Answer:
Given power of each bulb = 100 W
Power of each fan = 60 W
Time t =10 hours each day
Power of 2 bulbs = 2 × 100 = 200 W
Power of 2 fans = 2 × 60 = 120 W
Total power = 200 + 120 = 320 W = \(\frac{320}{1000}\) = 0.32 KW
Time duration of consumption 10 hours per day per month
= 10 hours × 30 = 300 hours
∴ Total energy consumed = Total power x Time duration
= 0.32 KW × 300 h = 96 KWh
∴ Total cost = 96 x 3 = ₹ 288.
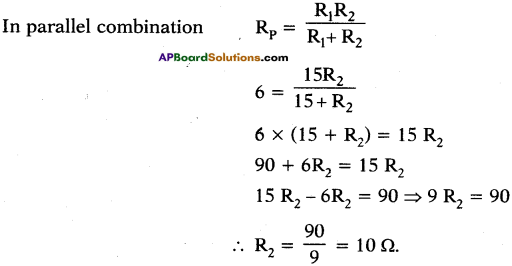
Question 35.
What resistance must be connected to a 15 Ω resistance to provide an effective resistance of 6 Ω?
Answer:
Given R1 = 15 Ω, R2 = ?, Effective resistance Rp = 6 Ω.
Since the effective resistance has decreased, R2 must be connected in parallel with R1
![]()
![]()

![]()
![]()

![]()
![]()