Andhra Pradesh BIEAP AP Inter 1st Year Zoology Study Material Lesson 6 Biology in Human Welfare Textbook Questions and Answers.
AP Inter 1st Year Zoology Study Material Lesson 6 Biology in Human Welfare
Very Short Answer Type Questions
Question 1.
Define parasitism and justify this term.
Answer:
An intimate association between two organisms of different species in which ‘one is benefited and the other one is often adversely affected’ is called parasitism. The word parasitism comes from the Greek word ‘parasitos’ (Para-at the side of Sitos – food or grain) which means one eating at another one’s table.
Question 2.
Distinguish between a vector and a reservoir host.
Answer:
| Vector host | Reservoir host |
| It is an organism that transfers the infective stages of parasites from one host to another. Ex: Anopheles for malaria parasite. | It is the host that lodges the infective stage of the parasite. It remains in the body till the main host is available. Ex: Monkey for Plasmodium. |
Question 3.
Distinguish between mechanical vector and biological vector.
Answer:
| Mechanical vector | Biological vector |
| It is the vector that merely transfers the infective stages of parasites without parasitic development. Ex: Housefly and cockroach for Entamoeba. | It is the vector in which the parasite undergoes a part of the development before it gets transferred. Ex: Female anopheles mosquito in the case of plasmodium. |
Question 4.
What is a hyperparasite? Mention the name of one hyper-parasite.
Answer:
A parasite that parasitizes another parasite is called a Hyper parasite.
Ex: Nosema notabilis (a cnidosporan) is a parasite in Sphaerospora polymorpha (a cnidosporan parasite in the urinary bladder of the toadfish).
![]()
Question 5.
What do you mean by parasitic castration? Give one example.
Answer:
Some parasites cause the degeneration of gonads of the host making it sterile. This effect is called parasitic castration.
eg: Sacculina (root-headed barnacle, a crustacean) causes the degeneration of ovaries in the crab Carcinus maenas.
Question 6.
What are the endo-parasitic adaptations observed in Fasciola hepatica?
Answer:
The life cycle of Fasciola hepatica (sheep liver fluke) is very complex involving many developmental stages and two intermediate hosts to increase the chances of reaching a new definitive host.
Question 7.
Define Neoplasia. Give one example.
Answer:
Some cause abnormal growth of the host cells in a tissue to form new structures. This effect is called Neoplasia which leads to cancers.
Ex: Some Viruses.
Question 8.
Define the most accurate definition of the term ‘health’ and write any two factors that affect health.
Answer:
Health is a state of complete physical, mental and social well-being and not merely the absence of any disease or absence of physical fitness. Our health may be affected by crenetic disorders, infections, and lifestyle.
Question 9.
Distinguish between infectious and non-infectious diseases. Give two examples each.
Answer:
| Infectious | Non-infectious |
| The diseases which are easily transmitted from one person to another are called infectious diseases. These are caused by pathogens. These are very common. Ex: Amoebic dysentery, Malaria, Elephantiasis, Typhoid. | The diseases which are not transmitted from one person to another and are not caused by pathogens are called non-infectious diseases. Ex: Genetic disorders, kidney problems. |
Question 10.
Entamoeba histolytica is an obligatory anaerobe justify.
Answer:
Mitochondria is absent in the endoplasm of Entamoeba histolytica. The absence of mitochondria indicates the obligate anaerobic nature of Entamoeba histolytica.
Question 11.
Distinguish between the precystic stage and the cystic stage of E.histolytica.
Answer:
| Precystic | Cystic |
| (i) It is a non-feeding, non-pathogenic stage. | (i) It is a feeding and infective stage. |
| (ii) It is small, oval, non motile form. | (ii) It is found in a round shape and surrounded by a delicate membrane. |
Question 12.
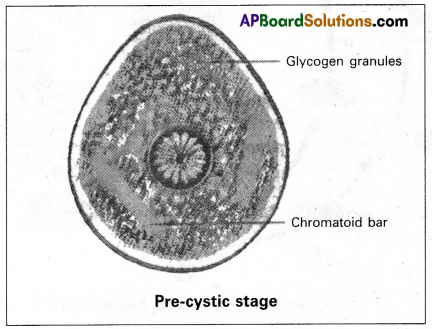
What is the reserve food in the precystic and early cyst stages of Entamoeba histolytica?
Answer:
The cytoplasm of the precystic stage stores glycogen granules and chromatoid bars (made of ribonucleic protein) which act as reserve food.
Question 13.
A person is suffering from bowel irregularity, abdominal pain, blood and mucus in stool, etc. Based on these symptoms, name the disease and its causative organism.
Answer:
- The disease is Amoebiasis.
- The causative agent is the Trophozoite of “Entamoeba histolytica”.
Question 14.
On the advice of a doctor, a patient has gone to a clinical laboratory for the examination of a sample of faeces. The lab technician, on observing the stool of the patient diagnosed that the patient was suffering from amoebiasis. Write any two characteristic features based on which the technician came to that conclusion.
Answer:
- Stool with blood and mucous.
- Presence of a tetra nucleated cyst.
![]()
Question 15.
Define ‘asymptomatic cyst passers’ with reference to Entamoeba histolytica.
Answer:
Some people do not exhibit any symptoms, such people are called carriers of asymptomatic cyst passers as their stand contains the tetranuclear cysts. They help in spreading the parasites to their persons.
Question 16.
What are the stages of plasmodium vivax that infect the hepatocytes of man?
Answer:
Sporozoite, Cryptozoite, Macrometacryptozoite.
Question 17.
Define the prepatent period. What is its duration in the life cycle of plasmodium vivax?
Answer:
The interval between the first entry of plasmodium into the blood in the form of sporozoites and the second entry of plasmodium into the blood in the form of Cryptozoics is called a prepatent period. It lasts approximately 8 days.
Question 18.
Define incubation period. What is its duration in the life cycle of Plasmodium vivax?
Answer:
The period between the entry of Plasmodium into the blood in the form of sporozoite and the first appearance of symptoms of malaria in man is called the incubation period which is approximately 10 to 14 days.
Question 19.
What are Schuffner’s dots? What is their significance?
Answer:
Small red coloured dots appear in the cytoplasm of the RBC known as Schuffner’s dots. These are believed to be the antigens released by the plasmodium (Malaria) parasites.
Question 20.
What are hemozoin granules? What is their significance?
Answer:
The malaria parasite digests the globin part of the ingested hemoglobin and converts the soluble heam into insoluble crystalline hemozoin. It is called the ‘malaria pigment’ which is a disposable product.
Question 21.
What is exflagellation and what are the resultant products called?
Answer:
Male gapnetes show lashing movements like flagella and get separated from the cytoplasm of microgametocyte. This process is called exflagellation and resultant products are called male (or) microgametes.
Question 22.
Why is the syngamy found in plasmodium called anisogamy?
Answer:
Since two gametes are dissimilar in size, the syngamy found in plasmodium is called anisogamy.
Question 23.
What is Ookinete? Based on the sets of chromosomes how do you describe it?
Answer:
Ookinete is a long, splendor, motile, vermiform, two sets of chromosomes are present in it. So it is described as diploid form.
Question 24.
A person is suffering from chills and shivering and high temperature. These symptoms are cyclically followed by profuse sweating and a return to normal body temperature. Based on these symptoms name the disease and its causative organism.
Answer:
The disease is malaria and the causative organism is Plasmodium vivax.
![]()
Question 25.
Describe the methods of biological control of mosquitoes.
Answer:
Introduction of larvivorous fishes like Gambusia, and insectivorous plants like Utricularia into the places where mosquitoes breed.
Question 26.
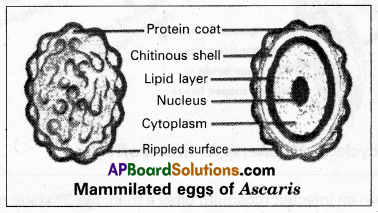
The eggs of Ascaris are called “mammillated eggs”. Justify it.
Answer:
Each egg of Ascaris is surrounded by a protein coat with a rippled surface. Hence the eggs of Ascaris are called “mammilated eggs”.
Question 27.
What is meant by nocturnal periodicity with reference to the life history of a nematode parasite you have studied?
Answer:
Microfilaria larvae of W.brancrofti migrate to the peripheral blood circulation during nighttime between 10 P.M – 4 A.M. This tendency is called nocturnal periodicity.
Question 28.
Distinguish between lymphadenitis and lymphangitis.
Answer:
| Lymphadenitis | Lymphangitis |
| Inflammation in the lymph glands is called lymphadenitis. | Inflammation in the lymph vessels is called lymphangitis. |
Question 29.
‘Elephantiasis is the terminal condition of filariasis’. Justify.
Answer:
Sweat glands of the skin in the affected region disintegrate and skin becomes rough so elephantiasis is the terminal condition of filariasis.
Question 30.
In which way does tobacco affect respiration? Name the alkaloid found in tobacco.
Answer:
Tobacco increases the carbon monoxide (CO) level and reduces the oxygen level in the blood. The alkaloid found in tobacco is “Nicotine”.
Question 31.
Define drug abuse.
Answer:
When drugs are used for a purpose other than medicinal use is called drug abuse.
Question 32.
From which substances ‘Smack’ and ‘coke’ are obtained?
Answer:
Smack is the common name for “Heroine”. It is obtained from the opium poppy plant. (Papaver somniferum).
Coke is the common name for “Cocaine”. It is obtained from the coca plant (Erythroxylum coca).
Question 33.
‘Many secondary metabolites of plants have medicinal properties. It is their misuse that creates problems. justify the statement with an example.
Answer:
Many secondary metabolites of plants like opioids, cannabinoids, and coca alkaloids are abused nowadays. Even though they have medicinal properties they cause some effects.
![]()
Question 34.
Why are cannabinoids and anabolic steroids banned in sports and games?
Answer:
These days some sports persons take drugs such as cannabinoids and anabolic steroids to enhance their performance (Doping) and abuse of such drugs also causes side effects that’s why such drugs are banned in sports and games.
Question 35.
Mention the names of any four drugs which are used as medicines to treat patients with mental illnesses like depression, insomnia, etc., that are often abused.
Answer:
Barbiturates, Amphetamines, Benzodiazepines, Lysergic aciddiethyl amides (LSD).
Short Answer Type Questions
Question 1.
What is the need for parasites to develop special adarptations? Mention some special adaptations developed by the parasites.
Answer:
Parasites have to evolve mechanisms to counteract and neutralize the host’s defence in order to be successful within the host. For this purpose, the parasites have developed many special adaptations such as the loss of unnecessary sensory organs, formation of organs for adhesion, high reproductive capacity, etc.
Parasitic adaptations: Parasites have evolved special adaptations to meet the requirements and lead successful lives in the hosts.
- In order to live in the host, some parasites have developed structures like hooks, suckers, rostellum, etc., for anchoring, e.g: Taenia solium.
- Some intestinal parasites have developed protective cuticles to withstand the action of the digestive enzymes of the host, e.g: Ascaris lumbricoides.
- Some intestinal parasites produce anti enzymes to neutralize the effect of the host’s digestive enzymes, e.g: Taenia solium.
- Some parasites live as obligatory anaerobes as the availability of oxygen is very rare for them, e.g: Entamoeba histolytica, Taenia solium, etc.
- Some intestinal parasites live as facultative anaerobes, i.e., if oxygen is not available, they live anaerobically and if oxygen is available, they respire aerobically, e.g: Ascaris lumbricoides.
Question 2.
Distinguish between hypertrophy and hyperplasia with an example for each.
Answer:
| Hypertrophy | Hyperplasia |
| It is an abnormal increase in the volume/size of the infected host cell caused by a parasite. e.g: R.B.C of a man infected by plasmodium. | It is an increase in the number of cells caused by parasites. e.g: Fasciola hepatica in the bile duct of sheep. |
Question 3.
Describe the structure of a trophozoite of Entamoeba histolytica.
Answer:
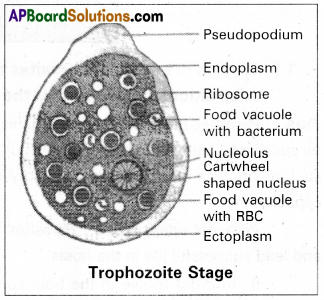
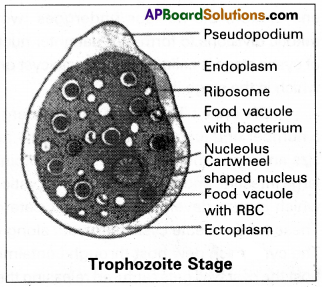
It is the most active, motile, feeding, and pathogenic stage that lives in the mucosa of the large intestine. It moves with the help of pseudopodium (lobopodium) which is produced anteriorly. The body of the trophozoite is surrounded by plasmalemma. Its cytoplasm is differentiated into an outer clear, viscous, non-granular ectoplasm and inner fluid-like granular, endoplasm.
Ribosomes, food vacuoles, and verticular cartwheel-shaped nucleus is present Absence of mitochondria indicates the obligate anaerobic nature of Entamoeba histolytica. It produces a proteolytic enzyme called histolytica which dissolves mucosas & sub-mucosa of the gut wall & releases blood, and tissue debris which are ingested by the trophozoite.
Hence food vacuoles are loaded with R.B.C & fragments of cells, and bacteria. The presence of R.B.C in the food vacuole and cart wheel-shaped nucleus are the characteristic features of the trophozoite.
![]()
Question 4.
Explain the life cycle of Entamoeba histolytica.
Answer:
The trophozoite undergoes binary fissions in the wall of the large intestine and produces a number of daughter entamoeba. They feed upon the bacteria and the hostly tissue elements, grow in size, and again multiply. After repeated binary fissions some of the young ones enter the lumen of the large intestine and transform into precystic stage.
Here, the precystic stage transforms into the cystic stage. Which in turn develops into tetranuclear cysts. The entire process is completed only in a few hours. These tetra nucleated cysts come out along with the faecal matter and can remain alive for about 10 days. These cysts reach new hosts through contaminated food and water. They pass into the small intestine of a new human host. Where the cyst wall gets, ruptured by the action of the enzyme trypsin releasing tetra nucleated amoeba. Such tetra nucleated exocyst amoeba is called metacyst. The four nuclei of the metacyst undergo mitotic divisions and produce eight nuclei. Each nucleus gets a bit of cytoplasm and thus eight daughter entamoeba are produced. The young ones develop into trophozoites and invade the large intestine.
Question 5.
Write a short note on the pathogenicity of Entamoeba histolytica.
Answer:
The trophozoites ‘dissolve’ the mucosal lining by histolysin going deep into the submucosa and causing ulcers. These ulcers contain cellular debris, lymphocytes, blood corpuscles, and bacteria. It leads to the formation of abscesses in the wall of the large intestine. Ultimately it results in stools with blood and mucous. This condition is called amoebic dysentery (or) Intestinal amoebiasis. Some people don’t exhibit any symptoms such people are called ‘carriers’ (or) asymptomatic cyst passers as their stools contain tetranucleotide cysts.
Question 6.
Describe the structure of the sporozoite of plasmodium vivax.
Answer:
The ultrastructure of the sporozoite of P. vivax was studied by barnham. It is sickle-shaped with a swollen middle part and pointed at both ends of it’s body. It measures about 15 microns in length and one micron in width. The body is covered by an elastic pellicle with microtubules which help in the curiggling movement of the sporozoite. The cytoplasm contains cell organelles such as the Golgi complex, E.R. mitochondria, and a nucleus.
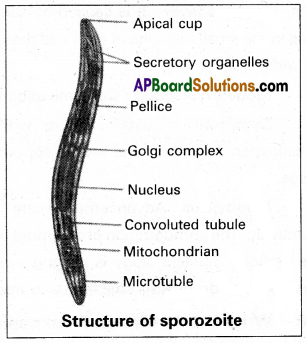
The cytoplasm also shows many convoluted tubules of unknown function throughout the body. It contains a cup-like depression called an apical cup at the anterior end into which a pair of secretory organelles opens. They secrete a cytolytic enzyme which helps in the penetration of sporozoite into the liver cell.
Question 7.
Describe the cycle of Golgi in the life history of Plasmodium Vivax.
Answer:
It was first described by Camillo Golgi. Hence it is also called the Golgi cycle. This is initiated either by the trophozoites of the pre-erythrocytic cycle (or) the micro meta cryptozoites of the exo-erythrocytic cycle. In the fresh R.B.C, these stages assume the spherical shape and transform into trophozoite. It develops a small vacuole that gradually enlarges in size, and pushes the cytoplasm and nucleus to the periphery.
Now the plasmodium looks like a finger ring. Hence this stage is called the signet ring stage soon it loses the vacuole, develops pseudopodia, and becomes an amoeboid stage. With the help of pseudopodium, it actively feeds on the content of the R.B.C and increases in size. As a result, the R.B.C grows almost double the size. This process is called hypertrophy. The malaria parasite digests the globin part of the ingested hemoglobin and converts the soluble haem into insoluble Haemozine. It is called malaria pigment. During this stage, small red coloured dots appear in the cytoplasm of R.B.C known as “Schuffner’s dots’.
Now the parasite loses the pseudopodia and increases in size finally it occupies the entire R.B.C and becomes schizont. It undergoes schizogony and produces 12-24 erythrocytic merozoites. They are arranged in the form of a rose hence this stage is called the rosette stage. Finally, merozoites are released along with haemozoine into the blood.
![]()
Question 8.
Explain the pathogenicity of Wucheria bancrofti in Man.
Answer:
The infection causes filarial fever which is characterized by headache, mental depression, and an increase in the body temperature. In general, the infection of filarial worm causes inflammation effect in lymph vessels and lymph glands. Inflammation in the lymph vessels is called lymphangitis and that of lymph glands is called lymphadenitis. In the case of heavy infection, the accumulation of dead worms blocks the lymph vessels and lymph glands resulting in immense swelling of limbs, scrotum of males, and mammary glands in females. Fibroblasts accumulate in this tissue and form the fibrous tissue. In severe cases, the sweat glands of the skin in the affected region disintegrate and the skin becomes rough. This terminal condition is called elephantiasis.
Question 9.
Write short notes on typhoid fever and its prophylaxis.
Answer:
Typhoid fever: It is caused by salmonella typhi which is a gram-negative bacterium. It mainly lives in the small intestine of man and then migrates to other organs through blood. It can be confirmed by the Widal test.
Mode of infection: Contamination through food and water.
Symptoms: Sustained fever with high temperature upto 104°F. weakness, stomach pain, constipation, headache, and loss of appetite. Intestinal perforation and death may also occur in severe cases.
Prophylaxis: Advancements made in biological science have armed us to deal with many infections effectively. The immunization programme by the use of vaccines has enabled us to completely irradicate like typhoid. Biotechnology is making available never cheaper vaccines, and the discovery of antibiotics and various other drugs also enabled us to treat typhoid.
Question 10.
Write short notes on Pneumonia and its prophylaxis.
Answer:
Pneumonia: It is caused by gram-positive bacteria such as Streptococcus pneumonia and Haemophilus influenza. They infect the alveoli of the lungs in human beings.
Mode of infection: Contamination by inhaling the droplets/aerosols released by an infected person or even by sharing the utensils with an infected person.
Symptoms: The alveoli get filled with fluid leading to severe problems in respiration. In severe cases, the lips and fingernails may turn gray to bluish in colour.
Prophylaxis: Advancements made in biological science have armed us to deal with many infections effectively. The immunization programme by the use of vaccines has enabled us to completely irradicate pneumonia. Biotechnology is making available newer, cheaper vaccines, and the discovery of antibiotics and various other drugs also enabled us to treat pneumonia.
Question 11.
Write short notes on the common cold and its prophylaxis.
Answer:
Common cold: It is caused by a rhinovirus group of viruses. They infect the nose and respiratory passage but not the lungs.
Mode of infection: Contamination is by direct inhalation of the droplets resulting from cough or sneezes of an infected person or indirectly through contaminated objects such as pens, books, cups, door knobs, computer keyboards or mice, etc.
Symptoms: Nasal congestion, discharge from the nose, sore throat, hoar senses, cough, headache, tiredness, etc., which usually last for 3-7 days.
Prophylaxis: Advancements made in biological science have armed to deal with many infections effectively. The immunization programme by the use of vaccines has enabled us to completely irradicate like viral diseases common cold. Biotechnology is making available newer cheaper vaccines, the discovery of antibiotics and various other drugs also enabled use to treat viral diseases like the common cold.
Question 12.
Write short notes on ‘ringworm’ and its prophylaxis.
Answer:
Ringworm: It is one of the most common infectious diseases in man. It is caused by many fungi belonging to the genera, Microsporum, Trichophyton, and Epidermophyton. Heat and moisture help these fungi grow in the skin folds such as those in the groin or between the toes.
Mode of infection: Contamination is by using towels, clothes or combs of the infected persons or even from the soil.
Symptoms: Appearance of dry, scaly, usually round lesions accompanied by intense itching on various parts of the body such as skin, nails, and scalp.
Question 13.
What are the adverse effects of tobacco?
Answer:
Effect: Smoking increases the carbon monoxide (CO) level and reduces the oxygen level in the blood. Nicotine stimulates the adrenal gland to release adrenaline and nor-adrenaline into the blood. These hormones raise blood pressure and increase the heart rate. Smoking is associated with bronchitis, emphysema, coronary heart disease, and gastric ulcer and increases the incidence of cancers of the throat, lungs, urinary bladder, etc. Smoking also paves the way to hard drugs. Yet smoking is very prevalent in society, both among young and old-. Tobacco chewing is associated with an increased risk of cancer of the oral cavity.
![]()
Question 14.
Write short notes on opioids.
Answer:
Opioids: These are the drugs obtained from the opium poppy plant Papaver somniferous (vernacular name: Nallamandu mokka): They bind to specific opioid receptors present in our central nervous system and gastrointestinal tract. Some of them are morphine, heroin, etc.
Morphine: It is extracted from the dried latex of the unripe seed capsule (Pod) of the poppy plant. It occurs as colourless crystals or a white crystalline powder.
Mode of abuse: Generally it is taken orally or by injection.
Effect: It is effective as a sedative and painkiller. It is very useful in patients who have undergone surgery.
Heroin: It is a white, bitter, odourless, and crystalline compound, obtained by the acetylation of morphine. Chemically it is diacetylmorphine. It is commonly called a snack.
Mode of abuse: Generally it is taken by shorting and injection.
Effect: Heroin is a depressant and slows down body functions.
Question 15.
Write short notes on Cannabinoids.
Answer:
Cannabinoids: These are a group of chemicals obtained from the Indian temp, plant cannabis Sativa (vernacular name Ganjai mokka). They interact with cannabinoid receptors present in the brain. The flower tops, leaves, and the resin of this plant are used in various combinations to produce marijuana, hashish, charas, and ganja. These daufs, cannabinoids are being abused by even some sports – persons (doping).
Mode of abuse: These are generally taken by inhalation and oral ingestion.
Effect: Show their effects on the cardiovascular system of the body.
Question 16.
Write short notes on Cocaine.
Answer:
Coca alkaloid or cocaine: It is a white, crystalline alkaloid that is obtained from the leaves of the coca plant Erythroxylum coca, native to South America. It is commonly called coke or crack.
Mode of abuse: It is usually shorted.
Effect: It has a potent stimulating action on the central nervous system as it interferes with the transport of the neurotransmitter dopamine. Hence it produces a sense of euphoria and increased energy. Its excessive dosage causes hallucinations.
Question 17.
Why adolescence is considered a vulnerable phase?
Answer:
Adolescence: It is the time period between the beginning of puberty and the beginning of adulthood. In other words. It is the bridge linking childhood and adulthood. The age between 12-18 years is considered adolescence period. It is both a period and process during which a child becomes muture. It is accompanied by several biological and behavioural changes. Thus, adolescence is a very vulnerable phase of the mental and psychological development of an individual.
Question 18.
Distinguish between addiction and dependence.
Answer:
Addiction: It is a psychological attachment to certain effects such as euphoria. The most important thing one fails to realize it, the inherent addictive nature of tobacco, drugs, and alcohol, with the repeated use of TDA, the tolerance level of the receptors present in our body increases. Consequently, the receptors respond only to higher doses leading to greater intake and addiction. However, it should be clearly borne in mind that the use of TDA even once, can be a forerunner to addiction. Thus, the addictive potential of tobacco, drugs, and alcohol pull the users into a vicious circle leading to their regular use (abuse) from which they may not be able to get out. In the absence of any guidance or counseling, people get addicted and become dependent on them.
Dependence: It is the tendency of the body of manifest a characteristic unpleasant condition (withdrawal syndrome). The regular dose of drugs or alcohol is abruptly discontinued. The withdrawal syndrome is characterized by anxiety. Shakiness (tremors), nausea, and sweating may be relieved when regular use is resumed again. Dependence leads the patients to ignore all social norms.
![]()
Question 19.
‘Prevention is better than cure. justify with regard to TDA abuse.
Answer:
The age-old adage of prevention is better than cure holds true here also.
Some of the measures successful in the prevention and control of TDA abuse among adolescents are:
- Avoid undue parental pressure: Every child has his/her own choice. Capacity and personality. Parents should not force their children to perform beyond their capacity by comparing them with others in studies, games, etc.
- Responsibility of parents and teachers: They should look for the danger signs and counsel such students who are likely to get into the ‘trap’.
- Seeking help from peers: If peers find someone abusing drugs or alcohol immediately it should be brought to the notice of their parents or teachers so that they can guide them appropriately.
- Education and counseling: Educating and counseling the children to face problems, stress, and failures as a part of life.
- Seeking professional and medical help: A lot of help is available in the form of highly qualified psychologists, psychiatrists, and de-addiction and rehabilitation programmers.
Long Answer Type Questions
Question 1.
Explain the structure and life cycle of Entamoeba histolytica with the help of neat and labelled diagrams.
Answer:
Entamoeba histolytica (Gr. entos – within : amoiba – change histos – tissues ; lysis – dissolve) is a microscopic and monogenetic parasite that inhabits the large intestine and causes amoebic dysentery or amoebiasis in man.
It is cosmopolitan in distribution but more common in the tropical and subtropical regions of the world. It is common in the people of rural and densely populated urban areas wherever the hygienic conditions are poor.
Structure: Entamoeba histolytica passes through three distinct stages in its life cycle namely
- Trophozoite stage
- Precystic stage
- Cystic stage
(i) Trophozoit stage: It is the most active, motile, feeding, and pathogenic stage that lives in the mucosa and sub-mucosa membrane of the large intestine; It moves with the help of a lobopodium which is produced anteriorly. The body of the trophozoite is surrounded by plasma-lemma. Its cytoplasm is differentiated into outer clear, viscous non-granular ectoplasm and inner fluid like granular endoplasm.
Ribosomes, food vacuoles, and vesicular, cartwheel-shaped nuclei are present in the endoplasm. The absence of mitochondria indicates the obligate anaerobic nature of Entamoeba histolytica. It produces the proteolytic enzyme called histolysis due to which the species name histolytica was assigned to it. Due to the effect of this enzyme the mucosa and submucosa of the gut wall are dissolved releasing some amount of blood, and tissue debris that are ingested by the trophozoites. Hence the food vacuoles are with erythrocyte fragments of epithelial cells and bacteria. The presence of RBC in food vacuoles and cartwheel-shaped nuclei are the characteristic features of the trophozoites of Entamoeba histolytic.
(ii) Precystic stage: It is the non-feeding and non-pathogenic stage of Entamoeba histolytica that is found in the lumen of the large intestine. It is a small, spherical, or oval, non-motileform. The cytoplasm of the precystic stage stores glycogen granules and chromatid bars (made of ribonucleic protein) which act as reserve food.
(iii) Cystic stage: It is round in shape and is surrounded by a thin, delicate, and highly resistant cyst wall. It is found in the lumen of the large intestine. The process of development of the cyst wall is called encystation. Which is a means of tiding over the un¬favourable conditions that the parasite is going to encounter while passing to a new host. Soon after the encystation, the nucleus undergoes two successive mitotic divisions to form four daughter nuclei. This type of cystic stage is called tetranuclear cyst or mature cyst which is the stage infective to man.
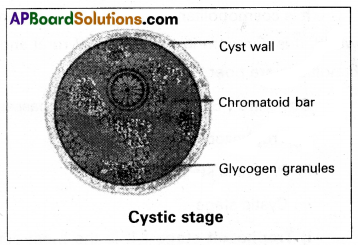
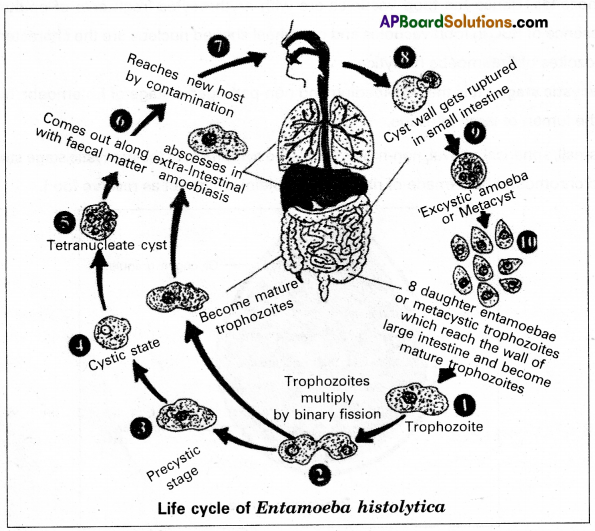
Life cycle: The trophozoites undergo binary fissions in the wall of the large intestine and produce a number of daughter entamoeba. They feed upon the bacteria and the host’s tissue elements, grow in size, and again multiply. After repeated binary fission some of the young ones enter of the lumen of the large intestine and transform into precystic stages. Here, the precystic stages transform into cystic stages which in turn develop into tetranuclear cysts. The entire process is completed only in a few hours. These tetranuclear cysts come out along with the faecal matter and can remain alive for about 10 days. The cyst reaches a new host through contaminated food and water. In the small intestine of a new human host, the cyst wall gets ruptured releasing the tetranuclear amoebae. Such tetranuclear excystic amoebae are called metacysts.
The four nuclei of the metacyst undergo mitotic divisions and produce eight nuclei. Each nucleus gets a bit of the cytoplasm and thus eight daughter entamoeba or metacystic trophozoites are produced. These young ones develop into feeding stages called trophozoites. They invade the mucous membrane of the large intestine and grow into mature trophozoites.
![]()
Question 2.
Describe the life cycle of plasmodium vivax in man.
Answer:
The life cycle of plasmodium in man (The human phase): In man, the plasmodium reproduces by asexual reproduction called schizogony. It occurs in liver cells (hepatocytes) as well as in RBC. In liver cells, it is called hepatic schizogony and in RBC it is called erythrocytic schizogony.
Hepatic Schizogony: This was discovered by short and Cranham. Whenever a mosquito infected by plasmodium bites a man, nearly 2000 sporozoites are released into the blood of man through its saliva, within half an hour, they reach the hepatocytes where they undergo pre-erythrocytic and exo-erythrocytic cycles.
Pre-erythrocytic cycle: Whenever the sporozoites enter the liver cells they transform into trophozoites. They feed on the contents of the hepatic cells, assume a spherical shape, and attain the maximum size. This stage is called the schizont stage. Its nucleus divides several times Mitotically, followed by the cytoplasmic divisions resulting in approximately 12,000 daughter individuals called cryptozoites or the 1st generation merozoites. They enter the sinusoids of the liver by rupturing the cell membrane of the schizont and the liver cells. This entire process is completed approximately in 8 days. Now, these first-generation merozoites have two options, i.e., they can enter either fresh liver cells and continue the exo-erythrocytic cycle or they can enter RBC and continue the erythrocytic cycle.
Exo-erythrocytic cycle: If the trophozoites enter the fresh liver cells, they undergo changes similar to that of the pre-erythrocytic cycle and produce the second generation merozoites called meta cryptozoites. These are of two types the smaller micro-metacryptozoites and larger macro-metacry- photosites. This entire process is completed approximately in two days. The macro-metacryptozoites attack fresh liver cells and continue another exo-erythrocytic cycle, whereas the micro-metacryptozoites always enter the bloodstream and attack fresh RBC to continue the erythrocytic cycle.
Prepatent period: The interval between the first entry of plasmodium into the blood in the form of sporozoites and the second entry of plasmodium into the blood in the form of Cryptozoic is called a prepatent period. It lasts approximately 8 days. During this period, the host does not show any clinical symptoms of the disease. It is only a means of multiplication.
Erythrocytic cycle: It was first described by Camillo Golgi Hence it is also called Golgi cycle. This cycle is initiated either by the trophozoites of the pre-erythrocytic cycle or the micro metocryptozoites of the exo-erythrocytic cycle in the fresh RBC, these stages assume the spherical shape and transform into trophozoites. It develops a small vacuole that gradually enlarges in size pushing the cytoplasm and nucleus to the periphery.
Now the plasmodium looks like a fisher ring. Hence this stage is called the signet ring stage. Soon it loses the vacuole, develops pseudopodia, and becomes an amoeboid stage with the help of pseudopodia. It actively feeds on the contents of the RBC and increases in size. As a result, the RBC grows almost double its size. This process is called hypertrophy. The malaria parasite digests the globin part of the ingested hemoglobin and converts the soluble haem into insoluble crystalline haemozoin. It is called the ‘malaria pigment’ which is called a disposable product. During this stage, small red coloured dots appear in the cytoplasm of the RBC known as Schaffner’s dots. These are believed to be the antigens released by the parasite.
Now the plasmodium loses the pseudopodia, further increases in size, occupies the entire RBC, and becomes a schizont. It undergoes schizogony similar to that of the pre-erythrocytic cycle and produces 12 to 24 erythrocytic merozoites. They are arranged in the form of the petals of a rose in the RBC. Hence this stage is called the rosette stage] Finally the erythrocyte bursts and releases the merozoites along with haemozoin into the blood. This cycle is completed approximately in 48 hours.
Incubation period: The period between the entry of plasmodium into the blood in the form of sporozoite and the first appearance of symptoms of malaria in man is called the incubation period, which is approximately 10 to 14 days.
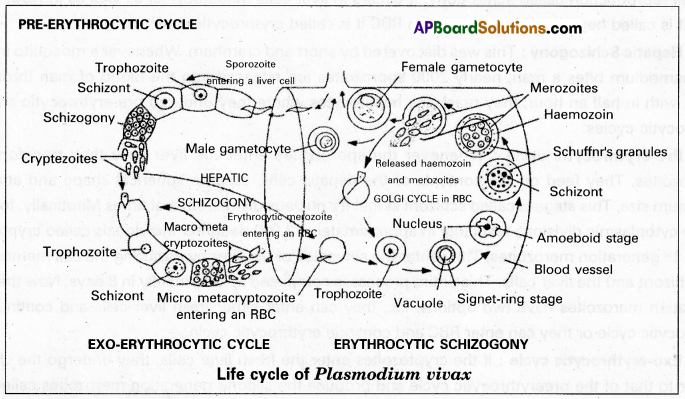
Formation of gametocytes: After repeated cycles of erythrocytic schizogony when the number of fresh RBC decreases, some merozoites enter the RBC and transform into gametocytes instead of continuing the erythrocytic cycle. This process generally takes place when the RBCs are present in the spleen and bone marrow.
The gametocytes are of two types namely smaller microgametocytes or male gametocytes and larger macrogametocytes or female gametocytes. The gametocytes cannot undergo further development in man as the temperature and PH of the blood man are not suitable for further development. These gametocytes reach the blood circulation and wait to reach the next host. They degenerate and die if they are not transferred to mosquitoes within a week.
Question 3.
Describe the life cycle of plasmodium vivax in mosquitoes.
Answer:
Life cycle of plasmodium in mosquito (The mosquito phase) Ross cycle: When a female Anopheles mosquito bite and sucks the blood of a malaria patient the gametocytes along with the other stages of the erythrocytic cycle reach the crop of mosquito. Here all the stages are digested except the gametocytes. Further part of the life cycle consists of
- Gametogony
- Fertilization
- Formation of ookinete & oocysts
- Sporogony
(i) Gametogony: The formation of male and female gametes from the gametocytes is called gametogony. It occurs in the lumen of the crop of mosquitoes.
Formation of male gametes: During this process, the nucleus of the microgametocyte divides into eight daughter nuclei called pronuclei which reach the periphery. The cytoplasm is pushed out in the form of eight flagella-like processes. Into each flagellum-like process, one pronucleus enters and forms a micro gamete or male gamete. These male gametes show lashing movements like flagella and get separated from the cytoplasm of microgametocyte. This process is called exflagellation.
Formation of female gamete: The female gametocyte undergoes a few changes and transforms into a female gamete. This process is called maturation. The nucleus of the female gamete moves towards the periphery and the cytoplasm at that point forms a projection. This projected region is called the fertilization cone.
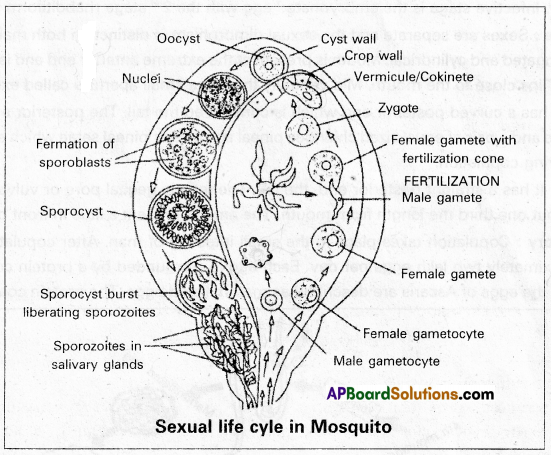
Fertilization: The fusion of male and female gametes is called fertilization. It also occurs in the lumen of the crop of the mosquito. When an actively moving male gamete comes into contact with the fertilization cone of the female gamete, it enters it, and the pronuclei and cytoplasm of these two gametes fuse with each other, resulting in the formation of a synkaryon Since the two gametes are dissimilar in size this process is known as anisogamy. The female gamete that bears the synkaryon is called the zygote which is round and non-motile.
(iii) Formation of ookinete and oocysts: The zygote remains inactive for some time and then transforms into a long, slender, motile, vermiform ookinete or vermicule within 18 to 24 hours. It pierces the wall of the crop and settles beneath the basement membrane. It becomes round and secretes a cyst around its body. This encysted ookinete is now called an oocyst. About 50 to 500 oocysts are formed on the wall of the crop and appear in the form of small nodules.
(iv) Sporogony: The formation of sporozoites in the oocysts is called sporogony. According to Bano, the nucleus of the oocyst first undergoes reduction division followed by repeated mitotic divisions resulting in the formation of about 1,000 daughter nuclei. Each bit of the nucleus is surrounded by a little bit of the cytoplasm and transforms into a sickle-shaped sporozoite. Oocyst with such sporozoites is called sporocyst.
When this sporocyst raptures, the sporozoites are liberated into the haemocoel of the mosquito. From there, they travel into the salivary glands and are ready for infection. The life cycle of plasmodium in mosquitoes completes in about 10 to 24 days.
![]()
Question 4.
Describe the structure and life cycle of Ascaris lumbricoides with the help of a neat and labelled diagram.
Answer:
Ascaris lumbricoides is commonly called the common roundworm. It lives in the small intestine of man, more frequently in children. It is cosmopolitan in distribution. The mode of infection is through contaminated food and water. The infective stage is the embryonated: egg with the 2nd stage rhabditiform larva.
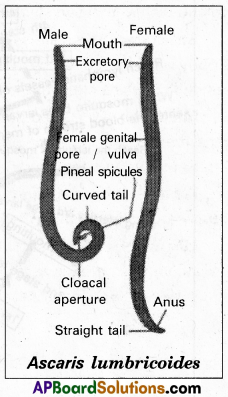
Structure: Sexes are separate and sexual dimorphism is distinct. In both males and females, the body is elongated and cylindrical. The mouth is present at the extreme anterior end and is surrounded by three chitinous lips close to the mouth. Mid ventrally there is a small aperture called an excretory pore.
Male: It has a curved posterior end which is considered the tail. The posterior end possesses a cloacal aperture and a pair of equal-sized chitinous pineal spicules or pineal setae which serve to transfer the sperms during copulation.
Female: It has a straight posterior end, the tail. The female genital pore or vulva is present mid-ventrally at about one-third the length from the mouth. The anus is present a little in front of the tail end.
Life history: Copulation takes place in the small intestine of a man. After copulation, the female releases approximately two lakh eggs per day. Each egg is surrounded by a protein coat with a rippled surface. Hence the eggs of Ascaris are described as mammilla eggs. The protein coat is followed by a chitinous shelf and a lipid layer internally. These eggs come out along with faecal matter. In the moist soil, development takes place inside the egg so that the 1st stage rhabditiform larva is produced. It undergoes the 1st moulting and becomes the 2nd stage rhabditiform larva which is considered the stage infective to man. They reach the alimentary canal of man through contaminated food and water.
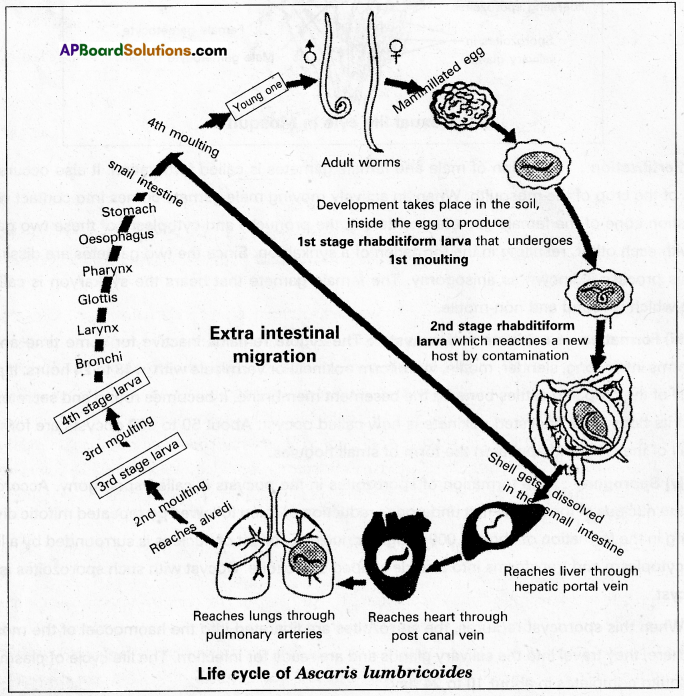
In the small intestine, the shell gets dissolved so that the 2nd stage larva is released. Now it undergoes extra-intestinal migration. First, it reaches the liver through the hepatic portal vein. From there it reaches the heart through the post caval vein. It goes to the lungs through the pulmonary arteries.
In the alveoli of the lungs, it undergoes the 2nd moulting to produce the 3rd stage larva. It undergoes the 3rd moulting so that the 4th stage larva is produced in the alveoli only. It leaves the alveoli and reaches the small intestine again through the bronchi, trachea, larynx, glottis, pharynx, oesophagus, and stomach. In the small intestine. It undergoes the 4th and final moulting to become a young one which attains sexual maturity within 8 to 10 weeks.
Question 5.
Describe the life cycle of wucheria bancrofti.
Answer:
Wucheria bancrofti is commonly called the filarial worm as it causes filariasis in human beings. It is a digenetic parasite that lives in the lymph vessels of man. Sir Patrick Manson identified the female culex mosquito as its secondary host.
Life cycle: It completes its life cycle in two hosts namely man and female culex mosquito.
In man: Both male and female worms are found coiled together in the lymphatic vessels of man. After copulation, the female releases the sheathed microfilaria larvae into the lymph of the man. Each sheathed microfilaria larva measures 0.2 to 0.3 mm in length. It is surrounded by a loose cuticular sheath which is supposed to be the modified shell. They migrate to the blood circulation and reside in the deeper blood vessels during the daytime. They move to the peripheral blood circulation during the nighttime between 10.00 pm and 4.00 am. This tendency is referred to as nocturnal periodicity. When a female culex mosquito sucks the blood of an infected person. They enter the gut of mosquitoes. They die if they are not transferred to mosquitoes within 70 days.
In mosquito: In the midgut of a mosquito, the sheath of the larva is dissolved within 2 to 6 hours of the infection. The ex-sheathed microfilaria larva penetrates the gut wall and reaches the heamocoel of the mosquito. From there, it reaches the thoracic muscles and transforms into a sausage-shaped larva within two days. It is called the first stage larva or first stage microfilaria. This undergoes two moultings within 10 to 20 days and transforms into infective 3rd stage microfilaria. It reaches the labium of the mosquito.
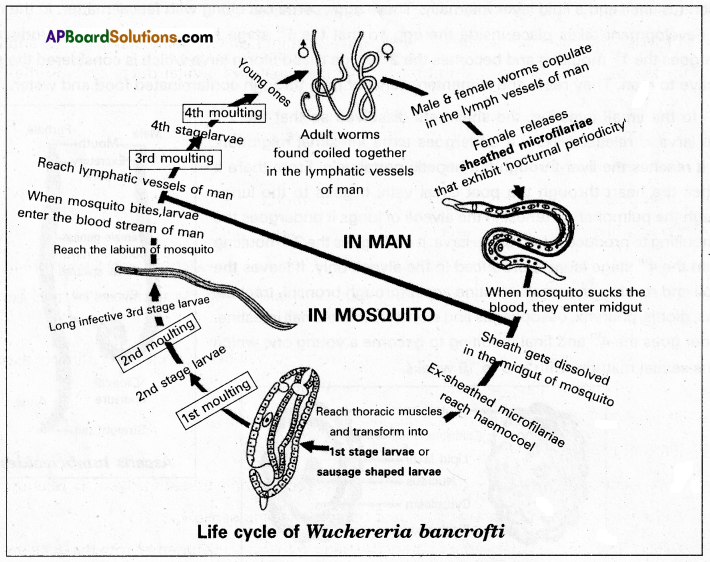
In man after the infection: When an infected mosquito bites a man, the 3rd stage microfilaria larvae enter the blood circulation of the man and finally reach the lymphatic vessels. Here they undergo the 3rd and the 4th moultings to produce young filarial worms. They attain sexual maturity within 5 to 18 months.










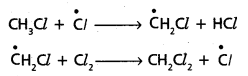
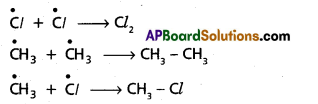
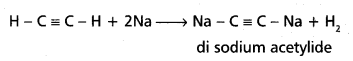
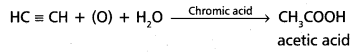
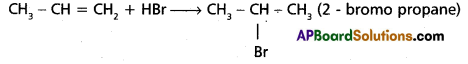
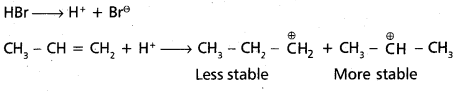
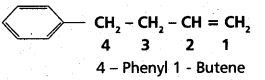
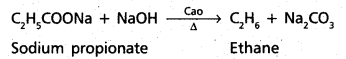
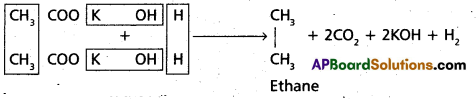
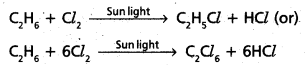



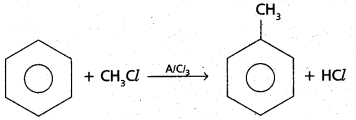
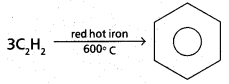
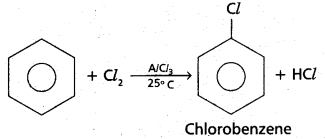
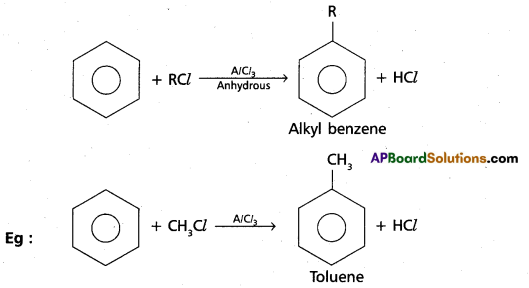
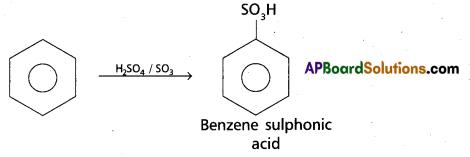
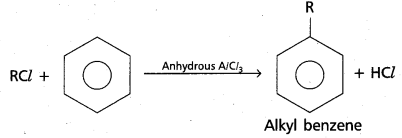
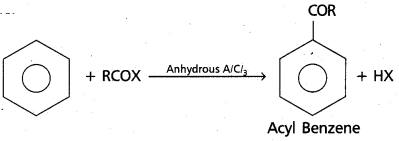
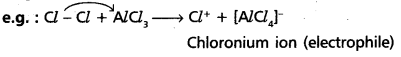
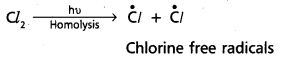
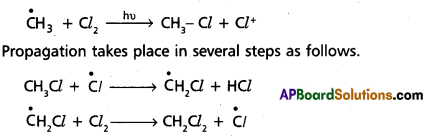
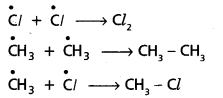
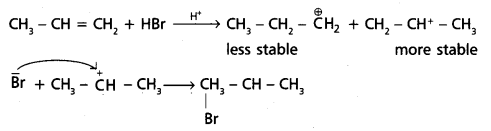
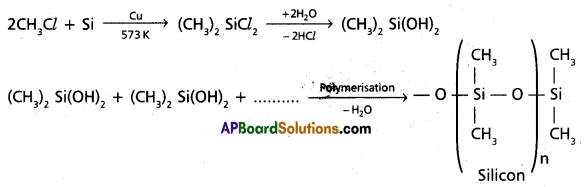
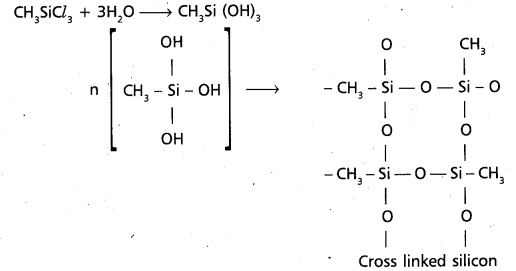
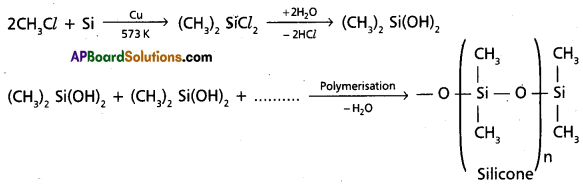
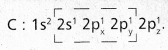
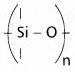 chains in which alkyl or phenyl groups occupy the remaining bonding positions on each silicon. They are hydrophobic (water repellant) in nature.
chains in which alkyl or phenyl groups occupy the remaining bonding positions on each silicon. They are hydrophobic (water repellant) in nature.