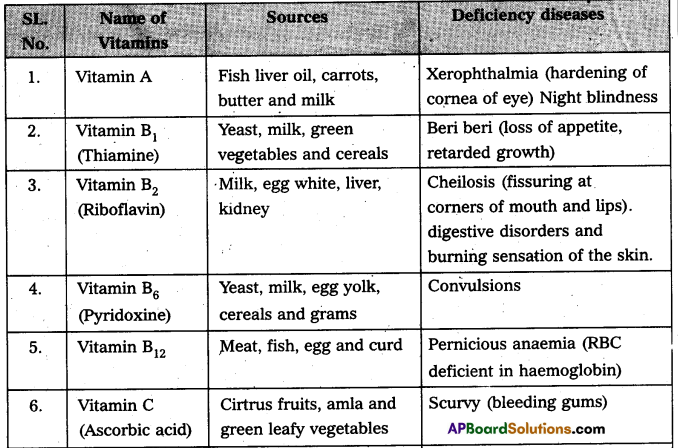
Andhra Pradesh BIEAP AP Inter 2nd Year Botany Study Material 8th Lesson Viruses Textbook Questions and Answers.
AP Inter 2nd Year Botany Study Material 8th Lesson Viruses
Very Short Answer Questions
Question 1.
What is the shape of T4 phage? What is its genetic material?
Answer:
Tad pole-shape. Its Genetic material is double-stranded DNA.
Question 2.
What are virulent phages? Give an example.
Answer:
T-even phages that attack the bacterium E.coli cause lysis of the cells and are called virulent phages Eg: Bacteriophage.
Question 3.
What is lysozyme and what is its function?
Answer:
Lysozyme is an viral enzyme, which breaks or lysed the bacterial cell wall to release their newly produced phage particles.
Question 4.
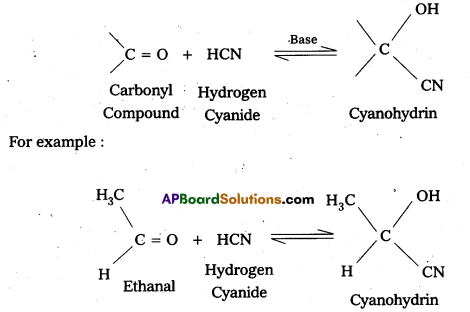
Define ‘lysis’ and ‘burst size’ with reference to viruses and their effects on host cells.
Answer:
The cycle in which the host cell wall ruptures to release the virions is called lysis. The number of newly synthesized bacteriophage particles released from a single affected host cell is called burst size.
![]()
Question 5.
What is a prophage?
Answer:
The phage DNA which is incorporated into bacterial DNA is called prophage.
Question 6.
What are temperate phages? Give an example.
Answer:
The phage DNA gets integrated into the circular bacterial DNA, becomes part of it and remains latent. Such phages are called temperate phages. Eg : A [Lambda] phage.
Question 7.
Mention the difference between virulent phages and temperate phages.
Answer:
| Virulent Phage | Temperate Phage |
| 1) Phages that attack the Bacterium E.coli cause lysis of the cells and are called virulent phages. | 1) The phage DNA gets integrated into the bacterial DNA, becomes part of it and remains latent are called temperate phages. |
| 2) They show lytic cycle. | 2) They show lysogenic cycle. |
Question 8.
What is the shape of Tmv? What is its genetic material?
Answer:
The shape of Tmv is rod and genetic material is single stranded RNA consisting of 6500 nucleotides.
Short Answer Questions
Question 1.
What is ICTV? How are viruses named?
Answer:
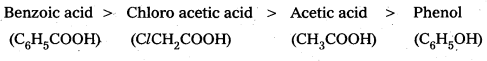
International Committee on Taxonomy of Viruses [ICTV] regulates the norms of classification and nomenclature of viruses. The ICTV has only three hierarchial levels, The family, Genus and Species. The family names end with the suffix viridae’ while the Genus name ends with virus and the Species names are common English expressions . Viruses are named after the disease they cause.
Eg : Polio virus.
![]()
Question 2.
Explain the chemical structure of viruses.
Ans. All viruses consist of two basic components, a core of nucleic acid that forms the genome and the surrounding coat of protein known as capsid. The capsid gives shape to virus and also protects the genome. Capsid is made up of protein sub-units called capsomeres.
The nucleic acid may be either a double stranded DNA (ds DNA) or single stranded DNA [ss DNA]. In general, viruses that infect plants have ss RNA and viruses that infect animals have ds DNA. Most viruses have a single nucleic acid molecule but a few have more than one. Eg: HIV has two identical RNA molecules.
Question 3.
Explain the structure of TMV.
Answer:
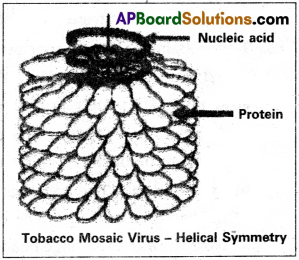
- It is a rod shaped virus. It is about’ 300 nm long and 18 or 19 nm in diameter with a molecular weight of 39 × 106 daltons.
- The capsid is made up of 2,130 sub-units called capsomeres.
- Capsomeres are arranged in a helical manner around a central core of 4 nm.
- Each protein sub-unit is made up of a single polypeptide chain with 158 amino acids.
- Single stranded RNA is present inside the capsid and is spirally coiled.
- RNA of TMV consists of 6,500 nucleotides.
Question 4.
Explain the structure of T-even Bacteriophages.
Answer:
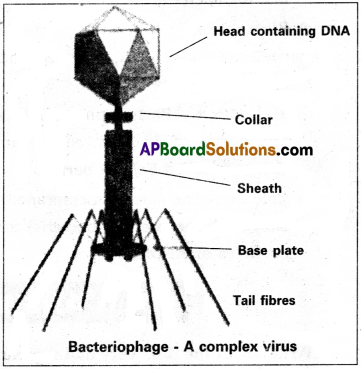
- The viruses which attack bacteria are called Bacteriophages. They were discovered by Twort (1915).
- Felix’d’ Herelle (1917) coined the term Bacteriophage.
- Bacteriophages are tadpole-shaped with a large head and a tail.
- The head is hexagonal and is capped by hexagonal pyramid, measures about 65 × 95 nm.
- The head is formed with several cap-someres, each of which is a single protein.
- The head protein forms a semipermeable membrane enclosing the folded double stranded DNA which is 1000 times longer than the phage.
- The tail is composed of several parts present around central core.
- The tail core is 95 nm long and 8 nm in diameter. It is surrounded by tail sheath composed of 144 sub-units which are arranged in 24 rings of 6 each.
- The head and tail are joined by collar whose function is not known.
- At the tip of the tail, hexagonal tail plate is present with six tail pins and tail fibres.
- The tail fibres help in attachment of the phage on to the host cell.
Question 5.
Explain the lytic cycle with reference to certain viruses.
Answer:
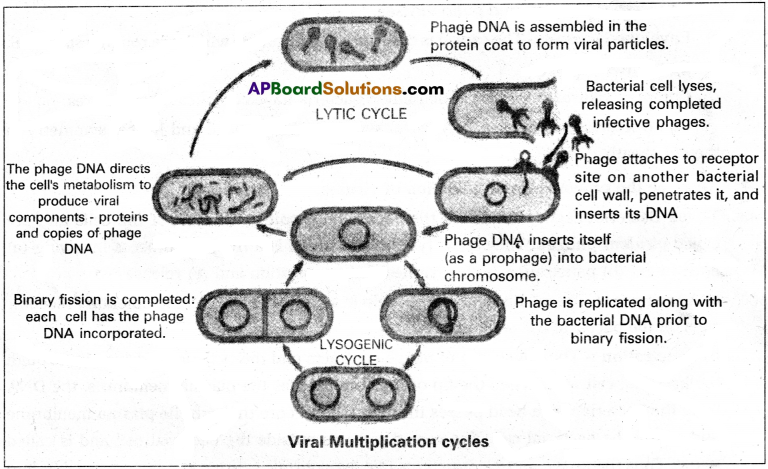
T-even Bacteriophages that attack the bacterium E.coli cause lysis of the cells and are called virulent phages. They show lytic cycle, which is a five step process involving (a) attachment (b) penetration (c) biosynthesis (d) maturation and (e) release
a) Attachment:
The tail fibres of phages help in attachment to the complementary receptor sites on the bacterial cell wall.
b) Penetration:
The tail sheath of phage contracts and the tail core is driven in through the bacterial cell wall. When the tip of the core reaches the plasma membrane, the DNA from the bacteriophage head passes through the tail Core through the plasma membrane and enters the bacterial cell. The capsid remains outside the bacterial cell and is called ghost. Thus the phage particle functions like hypodermic syringe and injects its DNA into the bacterial cell.
c) Biosynthesis:
When the phage DNA reaches the cytoplasm of the host cell, many copies of phage DNA, enzymes, and capsid proteins are synthesized using the cellular machinery of the host cell.
d) Maturation:
Bacteriophage DNA and capsids are assembled into complete virions. This period of time between the infection by a virus and the appearance of mature virions is called Eclipse period.
e) Release:
The plasma membrane of the host cell gets dissolved or lysed due to viral enzyme, lysozyme. The bacterial cell wall breaks, releasing the newly produced phage particles or virions.
Long Answer Questions
Question 1.
Write about the discovery and structural organization of viruses.
Answer:
In 1892, the Russian Pathologist Dmitri Iwanowski, while studying Tobacco mosaic disease, he filtered the sap of diseased tobacco leaf through filters and is injected into a healthy plant. He found the symptoms of Mosaic disease in it. Finally he reported that, a filterable agent was responsible for the disease. Later Martinus Beijerinck repeated Iwanowski’s experiments and concluded that, the disease causing agent was a contagious living fluid’ (contagium vivium fluidum).
W.M Stanley (1935) purified the sap and announced that the virus causing mosaic disease in tobacco could be crystallized and was named TMV. Fralnke conrael (1956) confirmed that the genetid material of TMV is RNA.
Structure:-
Viruses range in size from 300 nm as in TMV to 20 nm as in Parvoviruses. All viruses consist of two basic components, a core of nucleic acid that forms the genome and the surrounding coat of protein known as capsid. The capsid gives shape to the virus and provides a protective covering for the genome. It is made up of protein sub-units called, capsomeres. A virus contains its genetic information in either a double stranded (ds) DNA or single stranded (ss) DNA. In general, phytophages have ss RNA and zoophages have ds DNA. Bacteriophages are usually ds DNA viruses.
Shape:-
a) Helical viruses resemble long rods.
Eg: Rabies Virus, Tobacco Mosaic Virus.
b) Polyhedral viruses. They resemble polyhedral shape (many sided)
Eg : Herpes simplex and polio viruses.
c) Enveloped viruses: The capsid is covered by an envelope which are roughly spherical.
Eg : Influenza virus.
d) Complex viruses: Viruses which infect bacteria have complicated structures.
Eg : Bacteriophages have polyhedral symmetry in the head and helical symmetry in the tail sheath.
![]()
Question 2.
Describe the process of multiplication of viruses.
Answer:
T-even Bacteriophages that attack the bacterium E.coli cause lysis of the cells and are called virulent phages. They show lytic cycle, which is a five step process involving (a) attachment (b) penetration (c) biosynthesis (d) maturation and (e) release
a) Attachment:
The tail fibres of phages help in attachment to the complementary receptor sites on the bacterial cell wall.
b) Penetration:
The tail sheath of phage contracts and the tail core is driven in through the bacterial cell wall. When the tip of the core reaches the plasma membrane, the DNA from the bacteriophage head passes through the tail core through the plasma membrane and enters the bacterial cell. The capsid remains outside the bacterial cell and is called ghost. Thus the phage particle functions like hypodermic syringe and injects its DNA into the bacterial cell.
c) Biosynthesis:
When the phage DNA reaches the cytoplasm of the host cell, many copies of phage DNA, enzymes, and capsid proteins are synthesized using the cellular machinery of the host cell.
d) Maturation:
Bacteriophage DNA and capsids are assembled into complete virions. This period of time between the infection by a virus and the appearance of mature virions is called Eclipse period.
e) Release:-
The plasma membrane of the host cell gets dissolved hr lysed due to viral enzyme, lysozyme. The bacterial cell wall breaks, releasing the newly produced phage particles or varions.
Some Bacteriophages such as λ(Lambda) phages do not cause lysis and death of host cell when they multiply. Instead, the phage DNA, upon penetration into an E.coli cell gets integrated in to the circular bacterial DNA, becomes part of it and remains latent. Such phages are called temperate phages. The inserted phage DNA is now called prophages.
The prophages replicate along with the bacterial genetic material. The prophage remains latent with in the progny cells. In some rare spontaneous events, or when the host cell is exposed to UV light or some chemicals the phage DNA separates from the bacterial genetic material leading to the initiation of the lytic cycle. This lysogenic cycle facilitates the phenomenon of transduction.
Intext Questions
Question 1.
When discussing the multiplication of viruses, virologists prefer to call the process as replication, rather than reproduction. Why?
Answer:
Viruses must invade a host cell and take over the hosts metabolic machinery. So it is called replication.
![]()
Question 2.
In dealing with Public Health, the approach to deal with bacterial diseases is treatment. Can you guess the nature of the general Public Health approach to viral diseases? What example do you cite to support your answer?
Answer:
“Prevention is better than cure” – in AIDS.