Students can go through AP State Board 9th Class Physical Science Notes Chapter 6 Chemical Reactions and Equations to understand and remember the concept easily.
AP State Board Syllabus 9th Class Physical Science Notes Chapter 6 Chemical Reactions and Equations
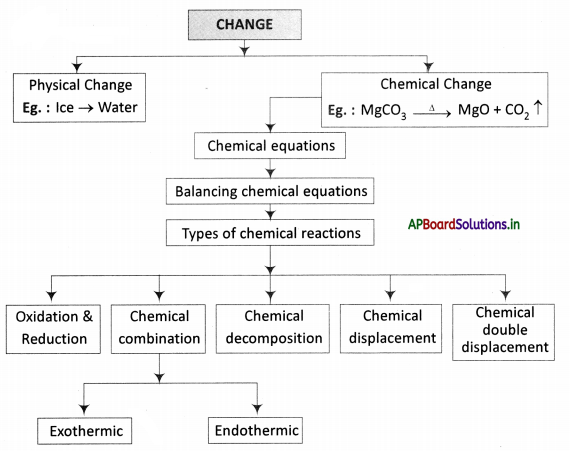
→ A chemical change is a permanent change.
→ In a chemical change, two substances react chemically to form new substances with different properties.
→ Chemical reactions are characterized by one or more of the following characteristics – the evolution of gas, change of color, formation of a precipitate, energy changes, and change of state.
→ A chemical equation represents a chemical reaction.
→ A complete chemical equation represents the reactants, products, and their physical state.
→ Reactions in which heat energy is absorbed by the reactants are endothermic reactions.
→ In exothermic reactions, heat energy is released by the reactants.
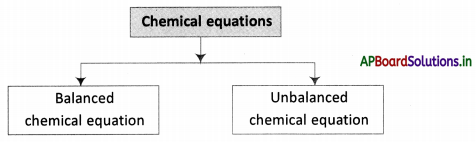
→ A chemical equation is balanced so that the number of atoms of each type involved in a chemical reaction is the same on the reactant and product sides of the equation.
→ Equations must always be balanced.
![]()
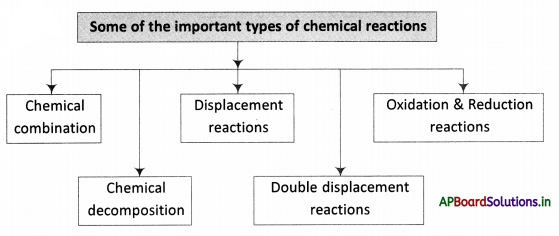
→ A combination reaction or synthesis is a reaction in which two or more substances combine to form a single substance.
→ A decomposition reaction is one in which a compound breaks up into two or more substances.
→ In a displacement reaction, one element replaces another element from a compound.
→ In a double decomposition reaction, two compounds react by exchanging their radicals.
→ Oxidation is a reaction involving the addition of oxygen or removal of hydrogen from a substance.
→ Reduction is a reaction involving the addition of hydrogen or the removal of oxygen from a substance.
→ Precipitation reactions produce insoluble salts.
→ According to the law of conservation of mass, “Mass can neither be created nor destroyed in a chemical reaction”.
→ The chemical equations are balanced to satisfy the law of conservation of mass in chemical reactions.
→ We should never change the formula of an element or a compound to balance an equation.
→ The process of making the number of different types of atoms equal on both sides of an equation is called the balancing of the equation.
→ All the combustion reactions are exothermic reactions.
→ The substance which gets oxidized is the reducing agent.
![]()
→ The substance which gets reduced is the oxidizing agent.
→ Two common effects of oxidation reactions that we observe in daily life are
- Corrosion of metals
- Rancidity of food
→ Corrosion causes damage to iron appliances.
→ When fats and oils are oxidized, they become rancid.
→ The precipitate is an insoluble substance.
→ Reactants: The substances which undergo chemical change in the reactions are called reactants.
→ Products: New substances formed in the reaction are called products.
→ Exothermic reaction: Reaction during which heat is released.
→ Endothermic reaction: Reaction in which heat is absorbed.
→ Chemical combination: It is a reaction in which two or more substances combine to form a single substance.
→ Chemical decomposition: It is a reaction in which a compound breaks up into two or more substances.
→ Displacement reaction: More active element replaces less active element from its compound in a chemical reaction.
→ Double displacement reaction: If two reactants exchange their constituents chemically and form two products, then the reaction is called a double displacement reaction.
→ Oxidation: It is a reaction involving the addition of oxygen or removal of hydrogen from a substance.
→ Reduction: It is a reaction involving the addition of hydrogen or removal of oxygen from a substance.
![]()
→ Corrosion: Chemical or electrochemical attack the surface of a metal.
→ Rancidity: The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called rancidity.
→ Antioxidants: Substances that prevent oxidation.
→ Chemical equation: A way of denoting a chemical reaction using the symbols for the participating particles (atoms, molecules, ions, etc.).
→ Chemical reaction: A change in which one or more chemical elements or compounds form new compounds.
→ Chemistry: The study of the elements and the compounds they form.
→ Oxidizing agents: Substances that oxidize other substances by providing oxygen to them or removing hydrogen from them.
→ Reducing agents: Substances that reduce other substances by removing oxygen from them or by supplying hydrogen to them.
→ Crystal: A solid in which atoms are arranged in a regular pattern.
→ Tarnish: To make something lose its shine and make it dull because of oxidation or rust.
→ Alloy: A material consisting of two or more metals or a metal and a non-metal. Ex: Steel.
→ Galvanizing: Iron or steel that has been coated with a layer of zinc to protect it from corrosion.
→ Rancid: Sour (or) Stale.
→ Redox reactions: The reactions in which both reduction and oxidation occur simultaneously are called redox reactions in short.
![]()
→ Exo: Outside.
→ Endo: Inside.
→ Thermo: Heat.
→ Respiration: When we inhale oxygen, it enters our body and combines with glucose in the cells of our body, and releases energy which helps to do the various works.
→ Henry Cavendish (1731 – 1810):
- Henry Cavendish conducted first his experiments on heat, electricity, and magnetism.
- He showed that water is composed of oxygen and hydrogen.
- He measured heats of fusion and evaporation as well as specific heats and those of the mixing of solutions in water.
- Cavendish’s measurements of the freezing points of various solutions showed the existence of compositions that yield maximum and minimum freezing points
- Cavendish’s experiments included the investigation of capacitance.
- In his experiments, he measured the strength of a current by shocking himself and estimating the magnitude of the pair.