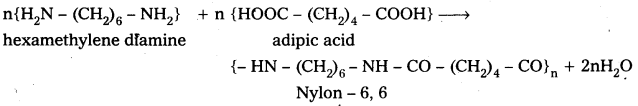
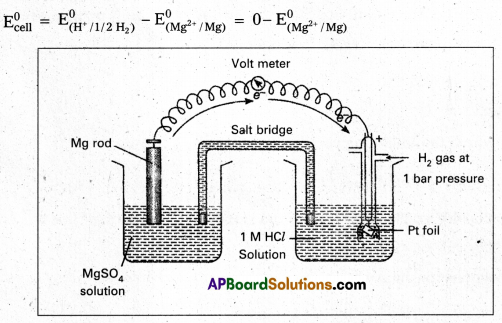
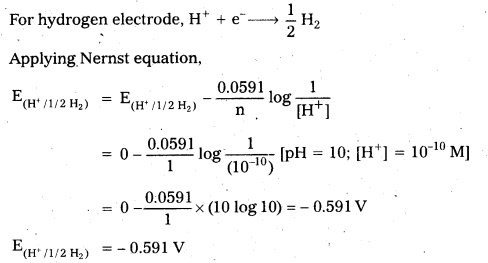
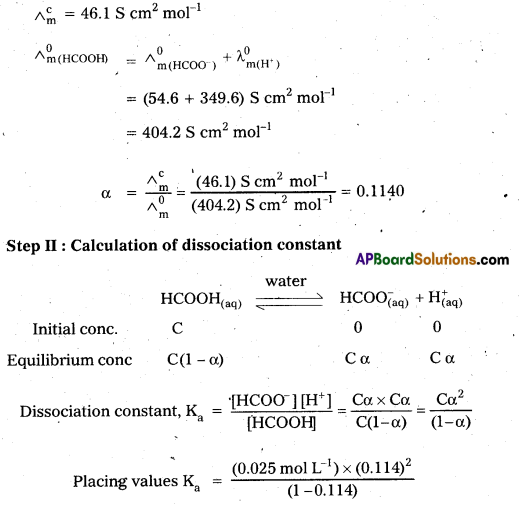
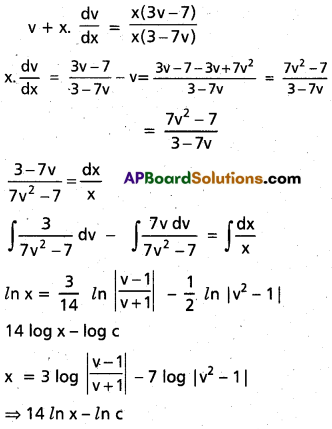
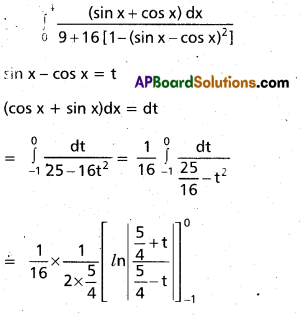
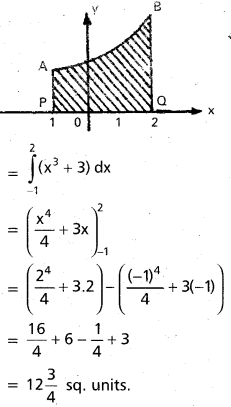
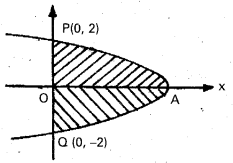
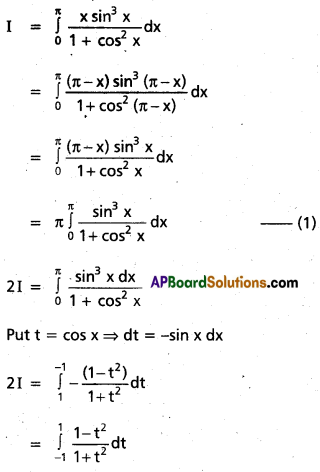
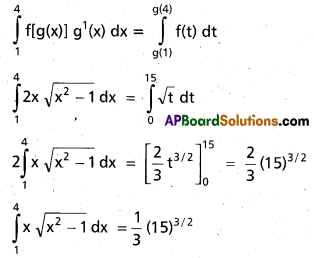
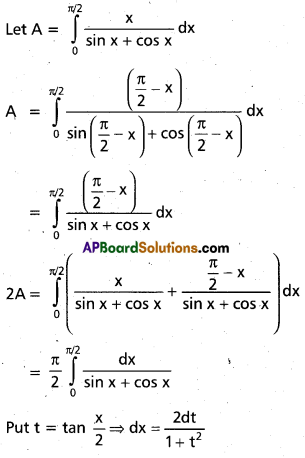
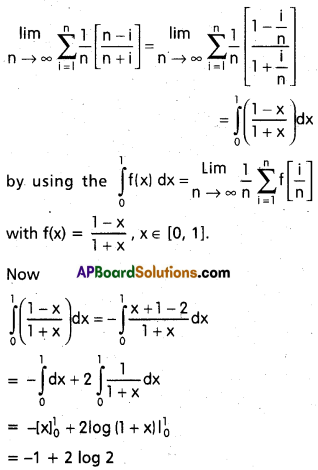
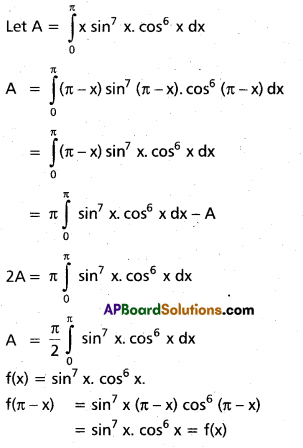
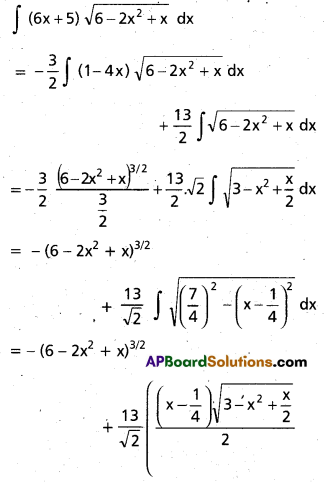
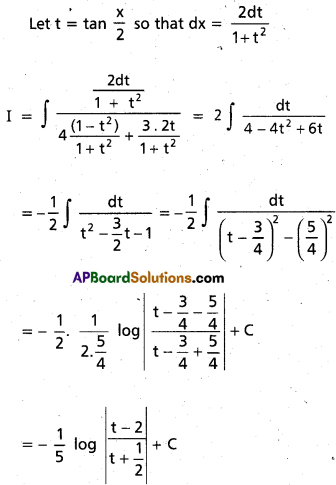
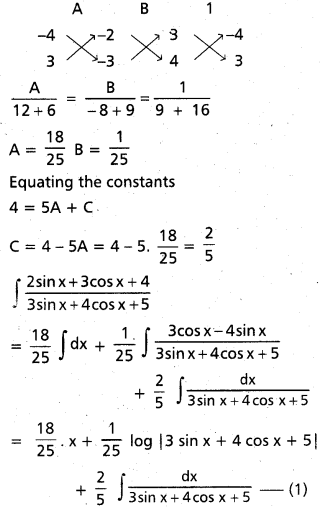
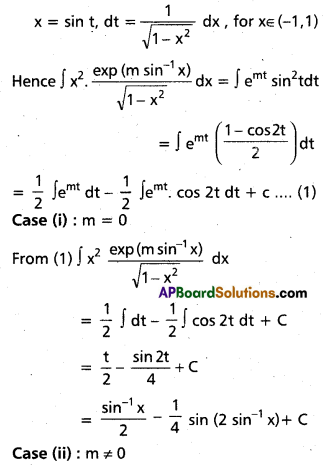
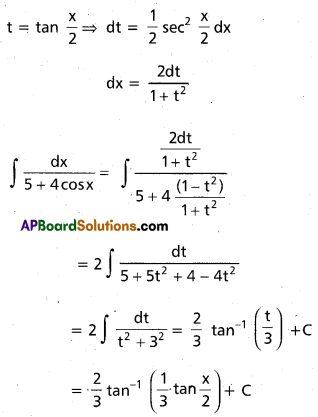
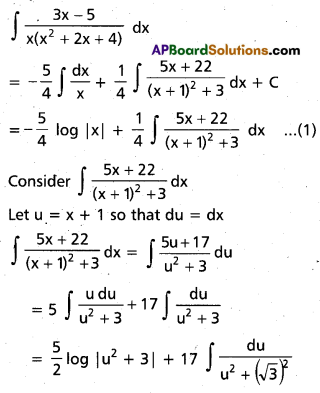
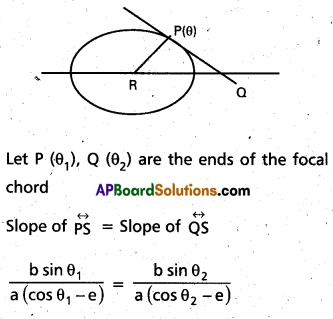
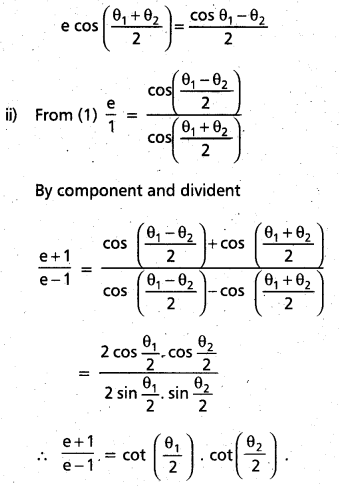
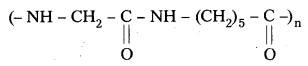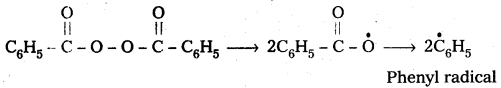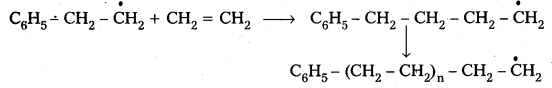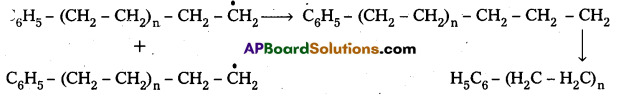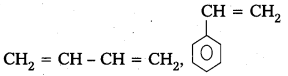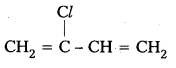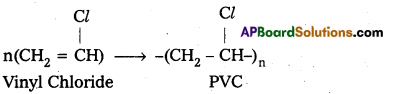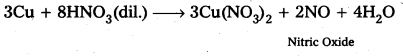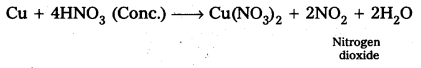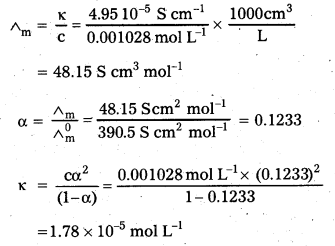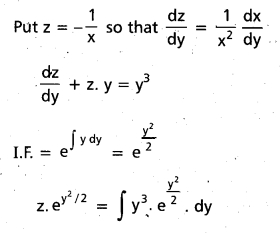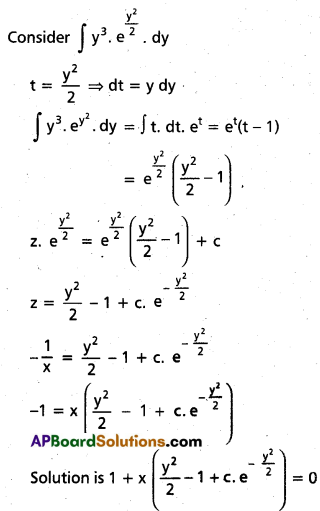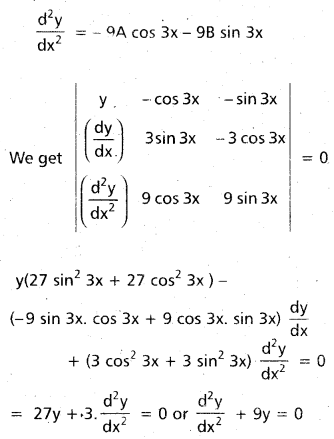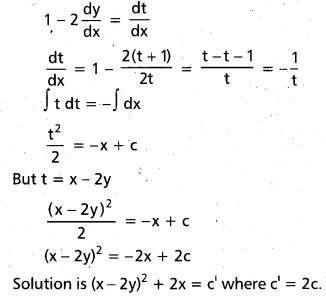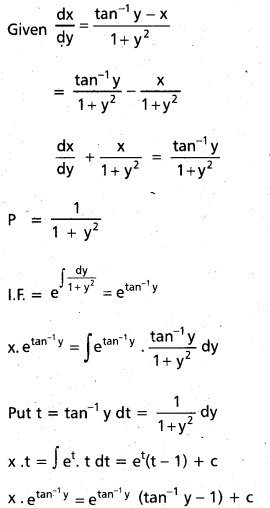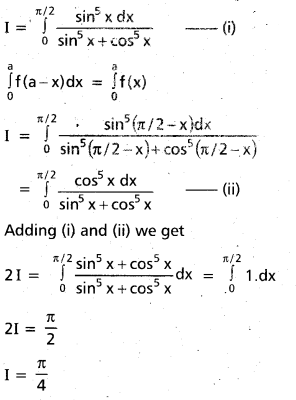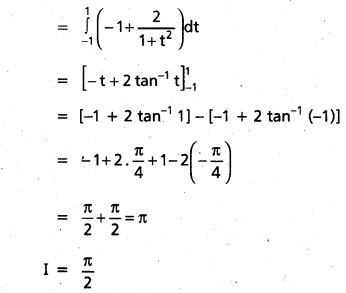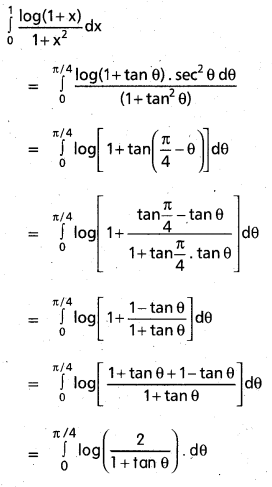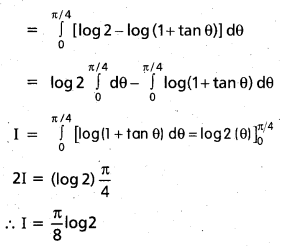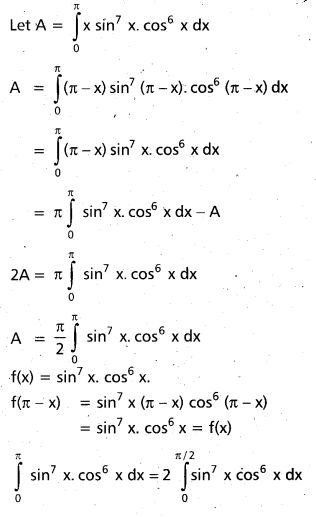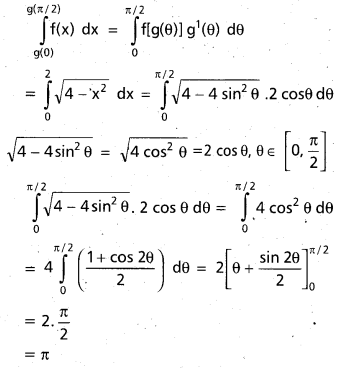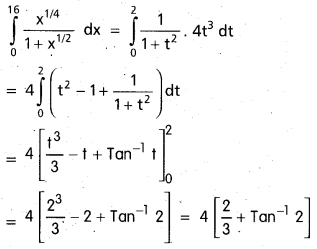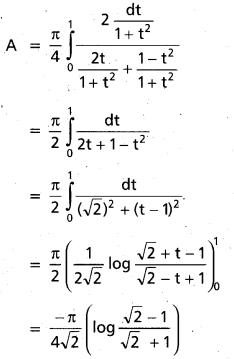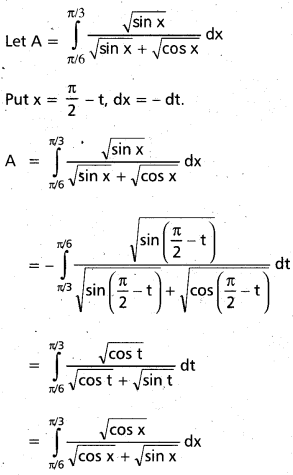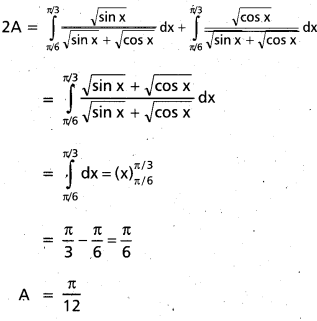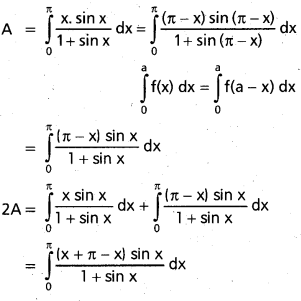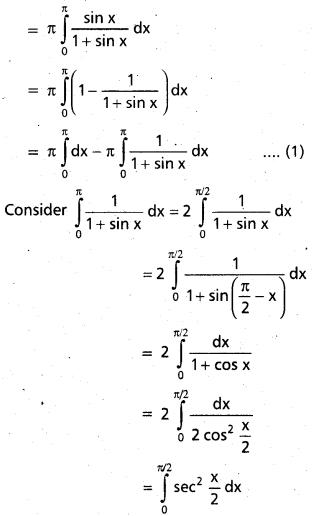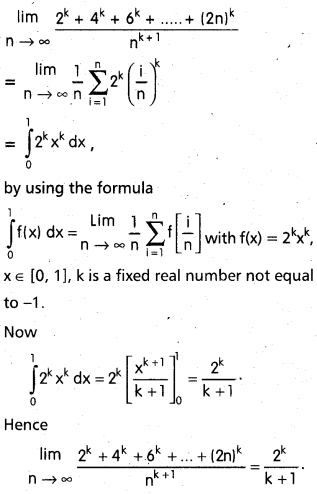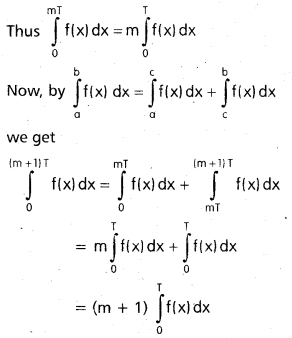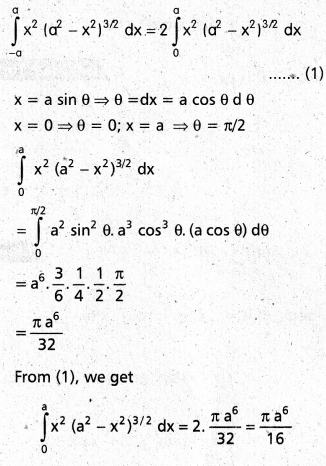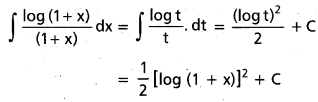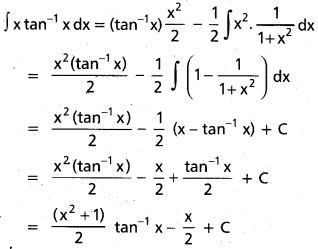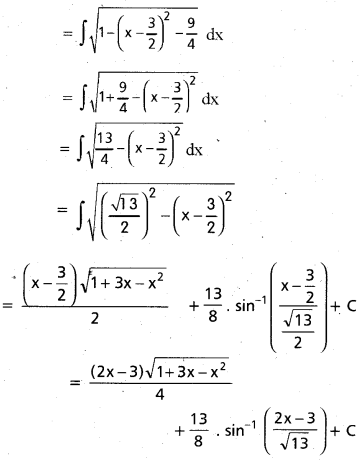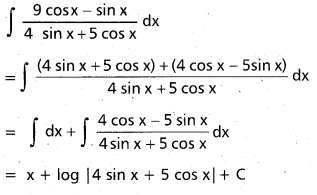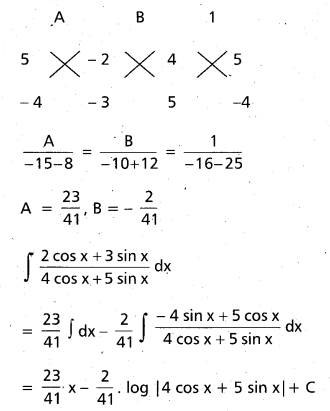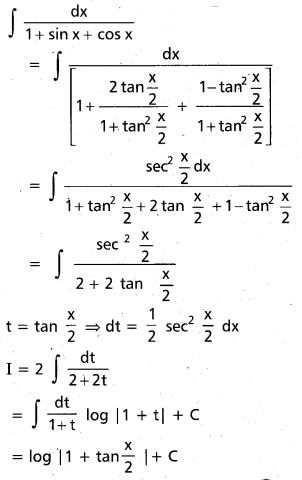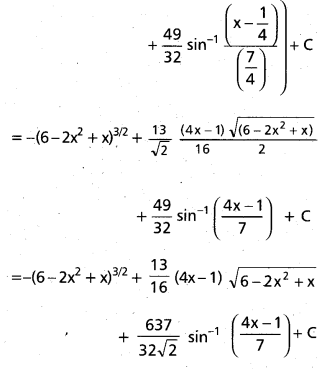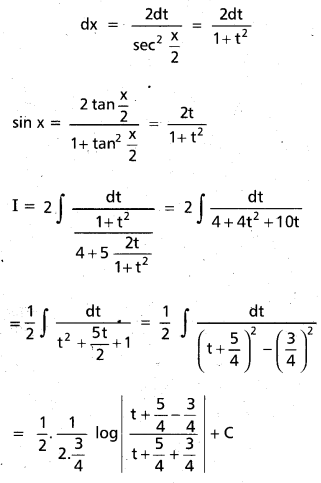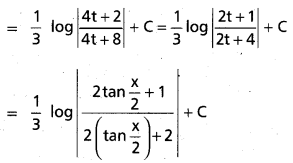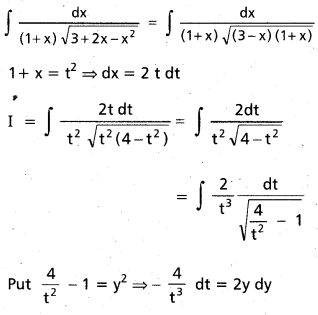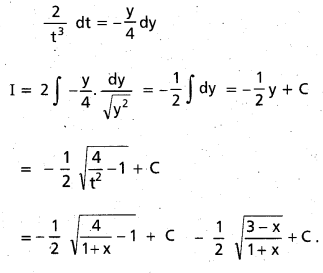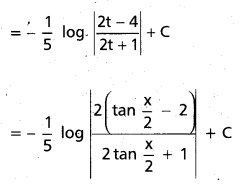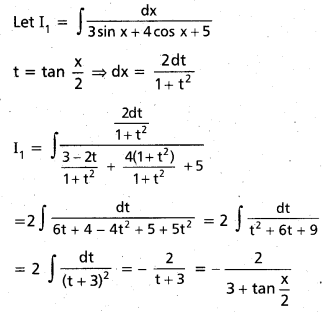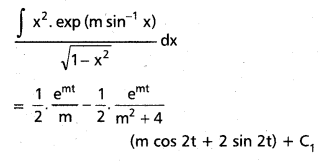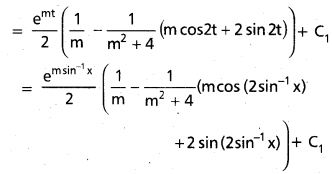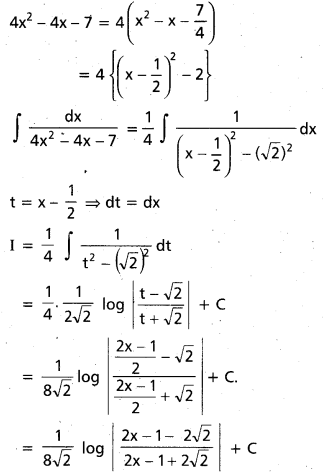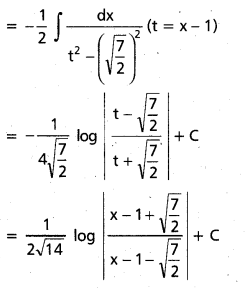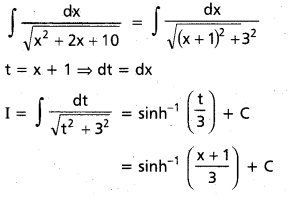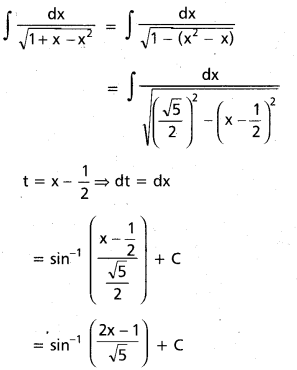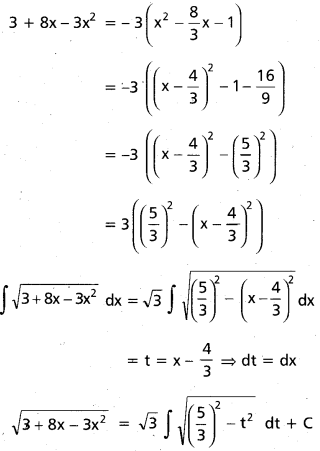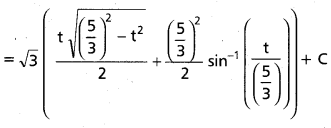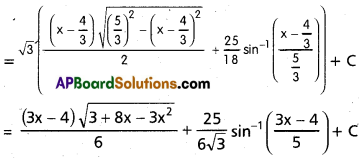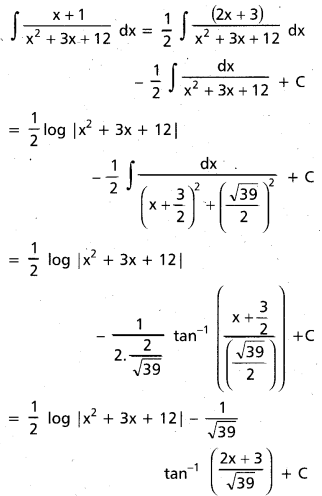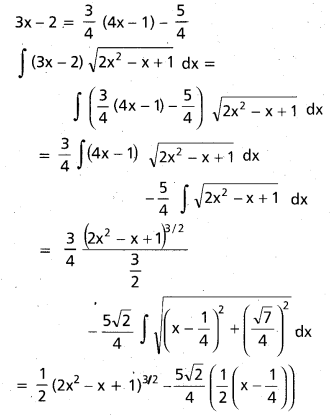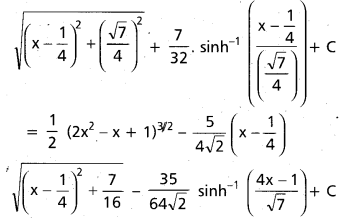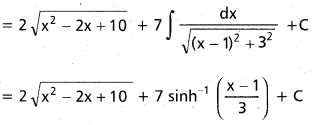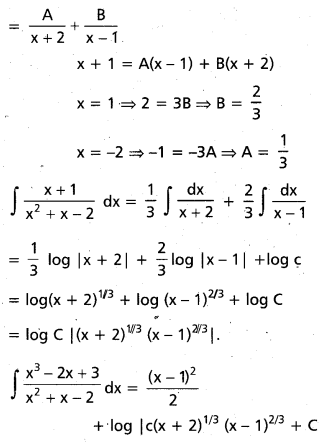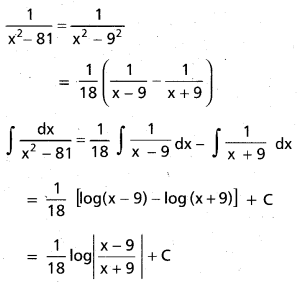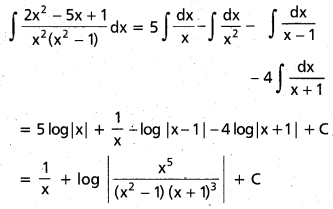Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material 9th Lesson Biomolecules Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material 9th Lesson Biomolecules
Very Short Answer Questions
Question 1.
Define Carbohydrates.
Answer:
The compounds which are primarily produced by plants and form a very large group of naturally occuring organic compounds are called Carbohydrates.
Eg: Glucose, Fructose, Starch.
-H> Carbohydrates are the polyhydroxy aldehydes (or) ketones.
Question 2.
Name the different types of carbohydrates on the basis of their hydrolysis. Give one example for each.
Answer:
On the basis of the hydrolysis, carbohydrates are classified as
- Monosaccharides, Eg : Glucose, fructose
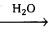 No saccharides
No saccharides - Oligosaccharides, Eg : Sucrose, maltose
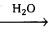 two monosaccharides
two monosaccharides - Polysaccharides, Eg : Starch, cellulose
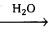 large number of monosaccharides.
large number of monosaccharides.
![]()
Question 3.
Why are sugars classified as reducing and non-reducing sugars ?
Answer:
Carbohydrates that reduce Fehling’s reagent, Tollen’s reagent are called reducing sugars.
Eg: Glucose.
Carbohydrates that doesnot reduce Fehling’s reagent, Tollen’s reagent are called non reducing sugars.
Eg : Sucrose.
Question 4.
What do you understand from the names
(a) aldo pentose and
(b) ketoheptose ?
Answer:
a) Aldo pentose : If a monosaccharide contains 5 carbon atoms with aldehyde group then it is known as aldo pentose.
b) Ketoheptose : If a monosaccharide contain seven carbons with a ketone group then it is called ketoheptose.
Question 5.
Write two methods of preparation of glucose.
Answer:
Methods of preparations of glucose :
1) From Sucrose.: Sucrose when boiled with di/.HC/ in alcoholic solution then glucose, fructose are obtained in equal portions.
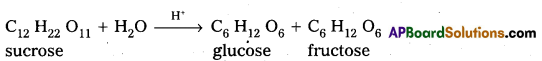
2) From Starch : Starch undergo hydrolysis, with dil. H2S04 at 393K under pressure to form glucose.
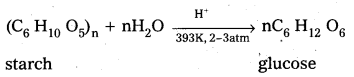
Question 6.
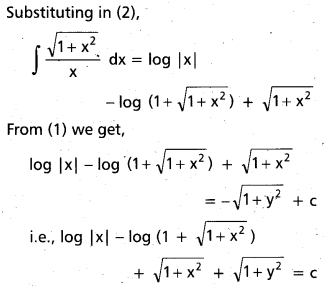
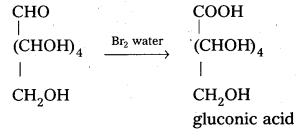
Glucose reacts with bromine water to give gluconic acid. What information do you get from this reaction about the structure of glucose ?
Answer:
Glucose reacts with bromine water to give gluconic acid. This reaction indicates that the carbonyl group present ip,glucose is an aldehyde group.
![]()
Question 7.
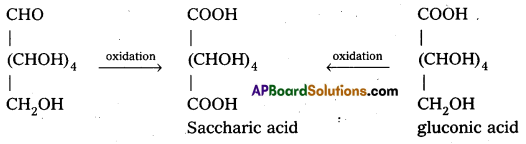
Glucose and gluconic acid on oxidation with nitric acid give saccharic acid. What Information do you get from this reaction about the structure of glucose ?
Answer:
Glucose and gluconic acid on oxidation with nitric acid give saccharic acid. This indicates the presence of primary alcoholic (-OH) group in glucose.
Question 8.
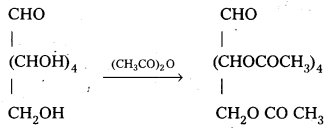
Glucose reacts with acetic anhydride to form penta acetate. What do you understand about the structure of glucose from this reaction ?
Answer:
Glucose reacts with acetic anhydride to form peta acetate. It confirms the presence of five- OH groups in glucose which are attached to different carbon atoms.
Question 9.
Give any two reasons to understand that glucose molecule has no open chain structure.
Answer:
Open chain structure of glucose could not explain
- why glucose doesnot respond to Schiffs test,
- why it does not react with NaHSO3 and NH3,
- why there is mutarotation.
Question 10.
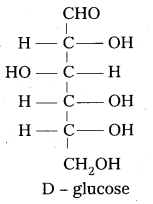
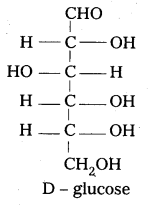
D – glucose means dextro rotatory glucose. Is it true ? Why ?
Answer:
In glucose the carbon atom attached to the lowest CH2-OH group is found to contain H atom on left side. So it is correlated to glyceraldehyde molecule and gives D-symbol. D-Glucose means not dextro rotatory glucose. D-symbol has nothing to do with optical activity of compound.
Question 11.
What are anomers ?
Answer:
Anomers : The two isomeric structures of a compound which differ in configuration at C-l only are called Anomers.
![]()
Question 12.
Write the ring structure of D-glucose. What are their names?
Answer:
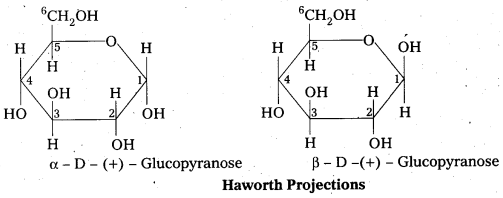
Question 13.
Write the ring structure and open chain structure of fructose.
Answer:
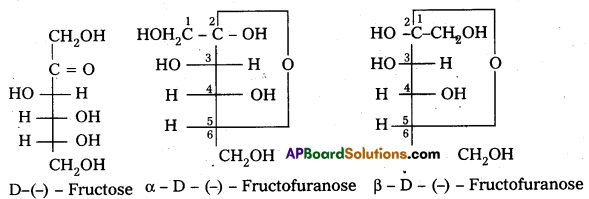
Question 14.
What do you understand by invert sugars?
Answer:
During the hydrolysis of sucrose there is a change in the sign of rotation, from dextro (+) to laevo (-) and the product is named as invert sugar.
Question 15.
What are amino acids? Give two examples.
Answer:
The organic compounds which contain amino (-NH2) functional group and carboxyl (-COOH) functional group are called amino acids.
Eg: Glycine, Alanine etc.
Question 16.
Write the structure of alanine and aspartic acid.
Answer:

![]()
Question 17.
What do you mean by essential amino acids ? Give two examples for non essential amino acids? [T.S. Mar. 16]
Answer:
Essential amino acids : The amino acids which cannot be synthesized in the body and must be obtained through diet are known as essential aminoacids.
Eg : valine,. leucine etc.
Examples of non essential amino acids are Alanine, glycine, Aspartic acid.
Question 18.
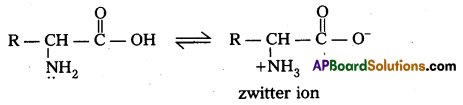
What is zwitter ion ? Give an example.
Answer:
Zwitter ion : In aqueous solution of amino acids, the carboxyl group can lose a proton and amino acid can accept that proton to form a dipolar ion. This ion is called as zwitter ion.
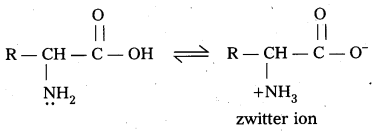
Question 19.
What are proteins ? Give an example.
Answer:
Proteins : A poly peptide with more than hundred amino acid residues, having molecular mass higher than 10,000 units is called a protein.
Eg : keratin, myosin, insulin.
Question 20.
What are fibrous proteins ? Give examples.
Answer:
When the poly peptide chains run parallel and are held together by hydrogen and disulphide bonds then fibre – like structure is formed. These are called fibrous proteins. These are insoluble in water.
Eg : keratin, myosin.
Question 21.
What are globular proteins ? Give examples.
Answer:
When the chains of polypeptides coil around to give a spherical shape then globular proteins are formed. These are usually soluble in water.
Eg : insulin and albumins.
![]()
Question 22.
How are proteins classified with respect to peptide bond ?
Answer:
With respect to peptide bond proteins classified into two types.
- Fibrous proteins
- Globular proteins.
Question 23.
What are the components of a nucleic acid ?
Answer:
- Nucleic acids are long chain polymers of nucleotides i.e poly nucleotides,
- Nucleic acids are constituted by pentose sugar, phosphoric acid, and nitrogenous hetero cyclic base (purine (or) pyrimidine).
Question 24.
Write the names of three types of RNA.
Answer:
Three types of RNA are :
- Messenger RNA (m – RNA)
- Ribosomal RNA (r – RNA)
- Transfer RNA (t – RNA).
Question 25.
Write the biological functions of nucleic acids.
Answer:
Biological functions of Nucleic acids :
- DNA is the chemical basis of heredity.
- DNA is the reserve of genetic information.
- DNA is capable of self duplication during cell division and identical DNA strands are transfered to daughter cells.
- RNA molecules are used in the protein synthesis in the cell
- The message for the synthesis of a protien is present in DNA.
![]()
Question 26.
Name the vitamin responsible for the coagulation of blood.
Answer:
The vitamin responsible for the coagulation of blood is vitamin K.
Question 27.
What are monosaccharides ?
Answer:
A carbohydrate which donot form any saccharide unit on hydrolysis (or) which cannot be hydrolysed is called monosaccharide.
Eg : glucose, fructose.
Question 28.
What are reducing sugars ?
Answer:
Carbohydrates that reduce Fehling’s reagent, Tollen’s reagent are called reducing sugars.
Eg : glucose.
Question 29.
Write two main functions of carbohydrates in plants.
Answer:
Carbohydrates are essential for life of plants.
- Carbohydrates are used as storage molecules as starch in plants.
- Cell wall of plants is made up of cellulose.
Question 30.
Classify the following into monosaccharides and disaccharides,
- ribose
- 2-deoxy ribose
- maltose
- fructose.
Answer:
- Ribose – Monosaccharide
- 2 – deoxy ribose – Monosaccharide
- Maltose – Disacch&ride .
- Fructose – Monosaccharide.
![]()
Question 31.
What do you understand by the term glycosidic linkage ?
Answer:
The linkage between two monosacharides of disacharides is called glycosidic linkage.
Question 32.
What is glycogen ? How is it different from starch ?
Answer:
The carbohydrate which is stored in animal body is called glycogen. It is also known as animal starch. Starch is a polysaccharide which is stored in plants where as glycogen is stored in animals (liver, muscles and brain).
- Glycogen mainly resembles amylopectin of starch in structure but glycogen is more branched than amylopectin.
Question 33.
What are the hydrolysis products of
i) sucrose and
ii) lactose ?
Answer:
i) The hydrolysis products of sucrose are glucose and fructose.
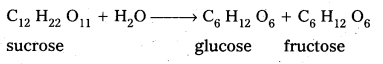
ii) The hydrolysis products of lactose are galactose and glucose.
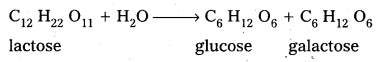
Question 34.
What is the basic structural difference between starch and cellulose ?
Answer:
In cellulose a straight chain polysaccharide composed only of β – D – glucose units which are joined by glycosidic linkage between C – 1 of one glucose unit and C – 4 of the next glucose unit.
Starch consists of two components – Amylose and Amylopectin. Amylose is a long un branched chain with 200 – 1000 α – D – glucose units held by C – 1 to C – 4 glycosidic linkage. Amylopectin is a branched chain polymer of α – D – glucose units in which Chain is formed by C – 1 to C – 4 glycosidic linkage where as branching occurs by C – 1 to C – 6 glycosidic linkage.
![]()
Question 35.
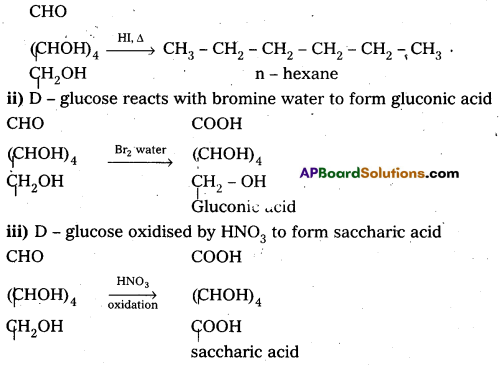
What happens when D – glucose is treated with the following reagents :
i) HI
ii) Bromine water
iii) HNOs.
Answer:
i) D – glucose on prolonged heating with HI, glucose forms n – hexane.
Question 36.
Enumerate the reactions of D – glucose which cannot be explained by its open chain structure.
Answer:
The following reactions cannot be explained by the open chain structure of D – glucose.
- Glucose has aldehyde group, but it does not respond to the reagents like Schiff s reagent, NaHSO3, NH3 etc., .
- The penta acetyl derivative of glucose doesnot react with hydroxyl amine.
- α and β – methyl glucosides cannot be explained by its open chain structure.
Question 37.
What are essential and non – essential amino acids ? Give one example for each.
Answer:
Essential amino acids : The amino acids that cannot be synthesised by the body and must be supplied in the diet are called essential amino acids.
Eg : valine, leucine, phenyl alanine etc.,
Non-essential amino acids : Other amino acids synthesised by the tissues of the body are called non-essential amino acids.
Eg : Glycine, alanine etc.,
Question 38.
Define the following as related to proteins :
i) Peptide linkage
ii) Primary structure
iii) Denaturation
Answer:
i) Peptide linkage :
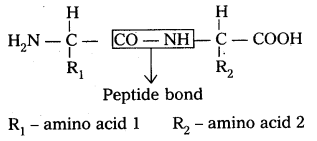
Amide  linkages between amino acids are known as peptide linkages. The product obtained from two amino acid molecules through peptide bond is called a dipeptide. Peptide chain may be extended to many amino acids.
linkages between amino acids are known as peptide linkages. The product obtained from two amino acid molecules through peptide bond is called a dipeptide. Peptide chain may be extended to many amino acids.
Proteins are naturally occuring polypeptides that contain more than 50 amino acid units.
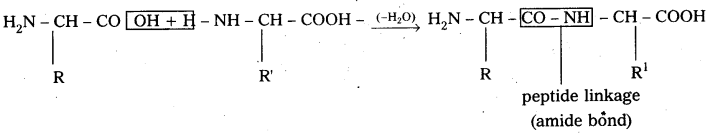
ii) Primary structure : The primary structure of protein is defined as the linear sequence of amino acid residues making up its polypeptide chain. Proteins may be formed of one or more polypeptide chains. The amino acid residues are linked by peptide bonds. The peptide bond is formed between carboxyl group of one amino acid and amino group of adjacent amino acid.
Eg : fibroin of silk.
iii) Protein Denaturation : The phenomenon of disorganization of native protein structure is known as denaturation. Denaturation results in the loss of secondary, tertiary nad quaternary structure of proteins. This involves a change in physical, chemical and biological properties of protein molecules.
Agents of denaturation:
Physical agents – Heat, violent shaking, X – rays, UV – radiation
Chemical agents – Acids, alkalies, organic solvents, urea, salts of heavy metals.
![]()
Question 39.
What are the common types of secondary structure of proteins ?
Answer:
Secondary structure: The secondary structure explains the shape of the polypeptide chain. It is derived from the primary structure by the formation of hydrogen bond interactions between amino acid residues.
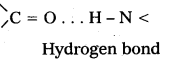
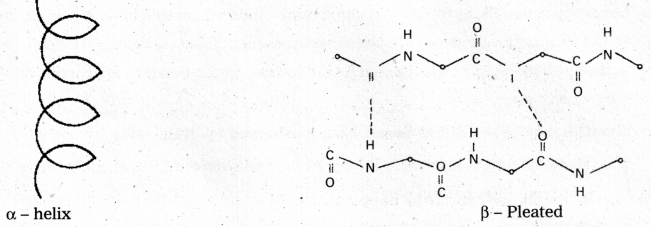
Disulfide bonds also occur either in same chain or between polypeptide chains. The secondary structure is classified into two types namely helical structure and pleated sheets, e.g.: Keratin of hair.
Question 40.
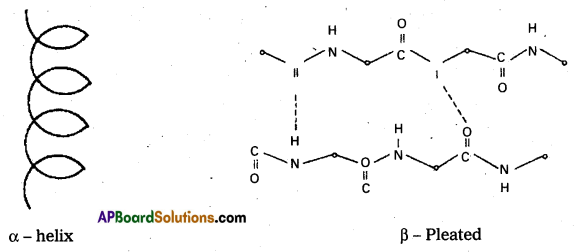
What type of bonding helps in stabilizing the α – helix structure of proteins ?
Answer:
The α – helix structure of proteins is stabilized by intramolecular hydrogen bonding between ![]() = O and
= O and ![]() – H groups of dlifferent peptide bonds.
– H groups of dlifferent peptide bonds.
Question 41.
Differentiate between globular and fibrous proteins.
Answer:
Globular proteins
- In this proteins the chains of poly peptides coil around to give a spherical shape
- These are soluble in water
- Eg: Insulin
Fibrous proteins
- In this proteins the poly peptides run parallel and are held together by hydrogens and disulphite – bondy
- These are insoluble in water
- Eg: keratin
![]()
Question 42.
How do you explain the amphoteric behaviour of amino acids ?
Answer:
Amino acids are the compounds having amino group (-NH2) i.e., a basic group and carboxyl group (-COOH) i.e., an acidic group
Zwitter ion,: In aqueous solution of amino acids, the carboxyl group can lose a proton and amino acid can accept that proton to form a dipolar ion. This is called as zwitter ion.
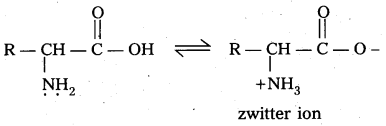
In zwitter ion form amino acids behave both acidic as well as basic nature. Therefore amino acids are amphoteric in nature.
Question 43.
Why are vitamin A and vitamin C essential to us ? Give their important sources.
Answer:
vitamin A and vitamin C are essential to us.
Explanation :
- Deficiency of vitamin A causes night blindness, red ness in eyes, xerophthalhia
- Deficiency of vitamin C causes pernicious anaemia (RBC deficient in haemoglobin)
Sources :
- Vitamin – A : Fish liver oil, carrots, butter and milk.
- Vitamin – C : Citrous fruits, amla, green leafy vegetables.
Question 44.
What are nucleic acids ? Mention their two important functions.
Answer:
- Nucleic acids are long chain polymers of nucleotides i.e poly nucleotides.
- Nucleic acids are constituted by pentose sugar, phosphoric acid, and nitrogenous hetero cyclic base (purine (or) pyrimidine).
Biological functions of Nucleic acids :
- DNA is the chemical basis of heredity.
- DNA is the reserve of genetic information.
- DNA is capable of self duplication during cell division and identical DNA strands are transfered to daughter cells.
- RNA molecules are used in the protein synthesis in the,cell.
- The message for the synthesis of a protien is present in DNA.
![]()
Question 45.
What is the difference between a nucleoside and a nucleotide ?
Answer:
Nucleosides: Nucleic acid bases combine with pentose sugars to form N – glycosides known as nucleosides.
Eg: Cytidine
Structure of Cytidine
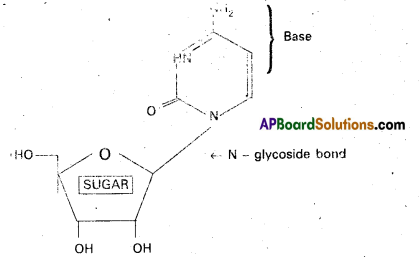
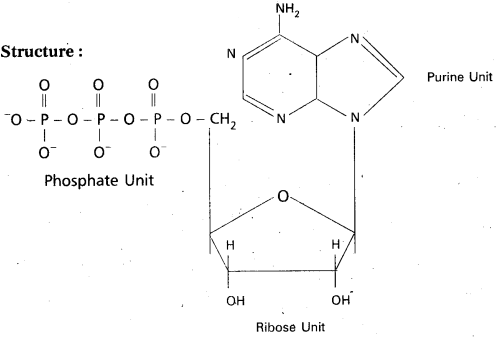
Nucleotides : Nucleotide is a phosphate ester of nucleoside and consists of a purine or pyrimidine base. Nucleotide contains a base moiety, a sugar moiety (i.e.,) a nucleoside moiety and phosphate group.
Nucleotide = base (purine / pyrimidine) + sugar (ribose/deoxyribose) + phosphate
Ex: Adenosine Triphosphate (ATP)
Short Answer Questions
Question 1.
How are the carbohydrates classified on the basis of their
a) Taste
b) Hydrolysis
c) Functional groups
Answer:
a) Taste : On the basis of their taste carbohydrates are classified into
i) Sugars, ii) Non sugars.
- Sugars : Carbohydrates which are sweet in taste.
Eg : Sucrose. - Non sugars : Carbohydrates which are not sweet in taste.
Eg : Cellulose.
b) Hydrolysis : On the basis of the hydrolysis, carbohydrates are classified as
- Monosaccharides, Eg: Glucose, fructose
 No saccharides
No saccharides - Oligosaccharides. Eg: Sucrose, maltose
 two monosaccharides
two monosaccharides - Polysaccharides, Eg: Starch, cellulose
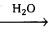 large number of monosacchandes.
large number of monosacchandes.
c) Functional groups: Carbohydrates classified into two types on the basis of functional group present.
- Aldoses: Carbohydrates having aldehyde group.
Eg: glucose. - Ketoses: Carbohydrates having ketone group.
Eg: fructose.
![]()
Question 2.
Write a brief note on the structure of glucose.
Answer:
1. The molecular formula of glucose is found to be C6H12O6. It can be written as CHO – (CHOH)4 – CH6OH’.
2. Acylation of glucose with acetic anhydride gives glucose pentacetate. Hence glucose molecule has five -OH groups.
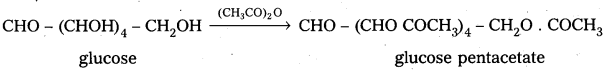
3. Glucose reacts with hydroxylamine and hydrogen cyanide forming corresponding oxime and cyano hydrin respectively. Hence glucose molecule has carbonyl group.
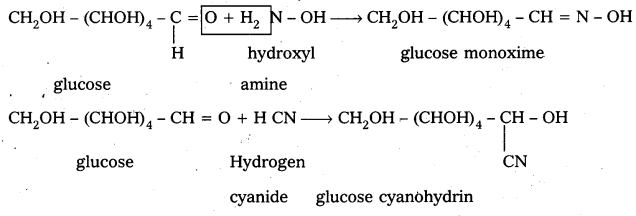
4. Glucose on oxidation with Tollens reagent and Fehling’s solution gives gluconic acid. Hence carbonyl group present in glucose is aldehydic group.
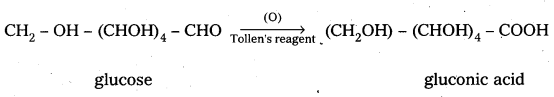
5. Glucose on oxidation with nitric acid gives glucaric (saccharic) acid. This suggests that glucose molecule has primary alcoholic group (-CH2OH).
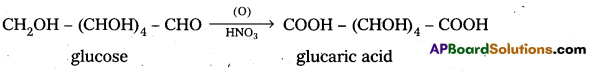
6. On reduction with HI, glucose gives n – hexane.
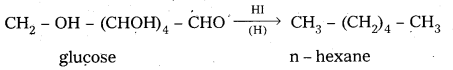
7. Open chain structure of glucose:
Based on above observations, glucose is given the open chain structure.
8. Objections against open chain structure of glucose :
Open chain structure of glucose could not explain
- Why glucose does not respond to schiffs test.
- Why it does not react with NaH SO3, and NH3.
- Why there is mutarotation.
Mutarotation :
Change in specific rotation of a compound from certain value to fixed value is known as “Mutarotation”.
The specific rotation of α – glucose is + 110° and that of β – form is + 19.7°. If each of them is separately dissolved in water and allowed to stand, their specific rotation gradually change and reach to a specific constant value + 52.5°.
9. Cyclic structure of glucose :
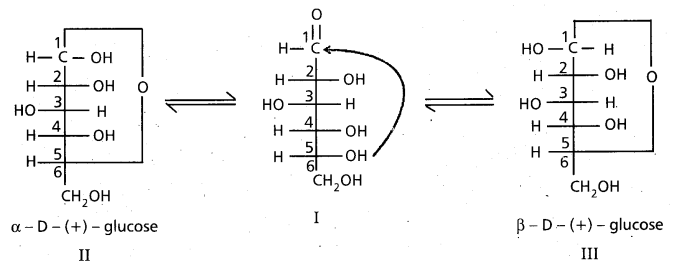
These two structures have difference in configuration at ‘C1‘ only and are called ANOMERS.
Cyclic structure of glucose explains all the properties of glucose.
10. Pyranose structure of glucose :
Question 3.
Write short notes on sucrose.
Answer:
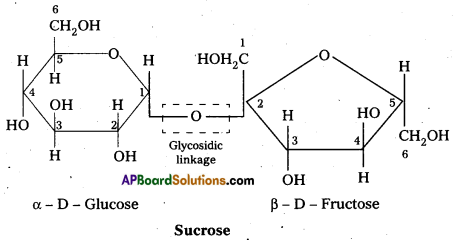
Sucrose is a disaccharide. It under go hydrolysis and forms equimolar mixture of D(+) glucose and D(-) fructose.
C12 H22 O11 + H12O → C6 H12 O6 (glucose) + C6 H12 O6 (fructose).
- In sucrose two monosaccharide units are held together by a glycosidic linkage between C – 1 of α – glucose and C – 2 of β – glucose.
- Sucrose is a non reducing sugar.
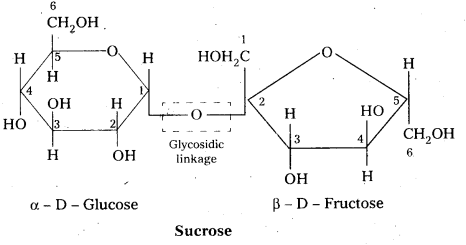
During the hydrolysis of sucrose there is a change in the sign of rotation, from dextro (+) to laevo (-) and the product is named as invert sugar.
![]()
Question 4.
Write the structure of maltose and lactose. What are the products of hydrolysis of maltose and lactose?
Answer:
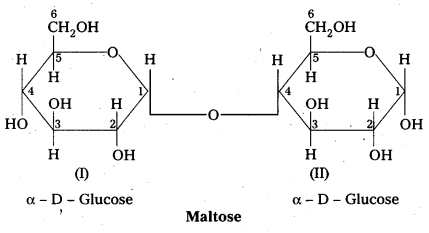
Structure of maltose:
- Maltose is formed from two α – glucose units (Disaccharide)
- C – 1 of one glucose linked to C -4 of another glucose
- It is a reducing sugar because C – 1 of 2nd glucose is solution have free aldehyde group.
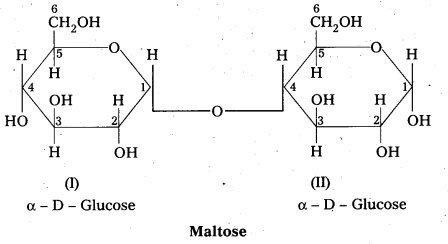
- maltose on hydrolysis to form two glucose units
C12 H22 O11 (Maltose) + H2O → C6H12O6 (glucose) + C6H12O6 (glucose)
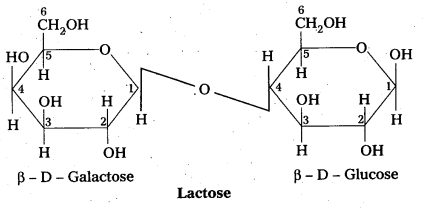
Structure of lactose:
- Lactose is milk sugar.
- Lactose is formed by the combination of β – glucose and β – galactose (Disaccharide)
- The linkage between C – 1 of galactose and c – 4 of glucose.
- It is a reducing sugar.
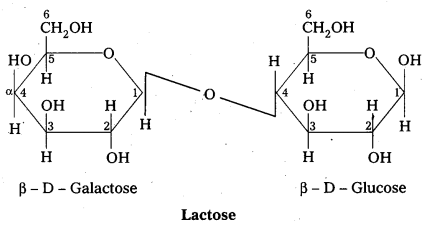
- Lactose on hydrolysis to form glucose and galactose
C12 H22 O11 (Lactose) + H2O → C6H12O6 (glucose) + C6H12O6 (galactose)
Question 5.
Write about polysaccharides with starch and cellulose as examples.
Answer:
Polysaccharides : The saccharides which on hydrolysis to form large number of monosaccharides are called polysaccharides.
Eg : Starch and cellulose Starch :
- Starch is the most important dietary source for human beings.
- Vegetables, roots, cereals are important sources of starch.
- It is a polymer of α – glucose.
- It is constituted by two components Amylose and amylopectin.
Amylose :
- It constitutes 15 – 20% of starch.
- Amylose is water soluble component.
- Amylose is a branched chain with 200 – 1000 α – D – glucose units held by C – 1 to C -4 glycosidic linkage.
Amylopectin:
- Amylopectin constitutes 80 – 85% of starch.
- It is a branched chain polymer of a – glucose units in which chain is formed by C – 1 to C – 4 glycosidic linkage whereas branching occurs by C – 1 to C – 6 glycosidic linkage.
Cellulose :
- ellulose occurs in plants and it is the most abundant organic substance.
- It is a major constituent of cell wall of plant cells.
- Cellulose is a straight chain polysaccharide composed only of β – D – glucose units which are joined by glycosidic linkage between C – 1 of one glucose and C – 4 of the next glucose.
![]()
Question 6.
Write the importance of carbohydrates.
Answer:
Importance of carbohydrates :
- Carbohydrates are essential for life of plants.
- Carbohydrates are used as storage molecules as starch in plants.
- Cell wall of plants is made up of cellulose.
- Carbohydrates are also essential for life of animals. Carbohydrates are used as storage molecules as glycogen in animals.
- Carbohydrate source hohey is used for a long time as an instant source of energy.
Question 7.
Explain the classification of proteins as primary, secondary, tertiary and quartemary proteins with respect ot their structure.
Answer:
Protein structure’s and shapes are studied at four different levels as
i) Primary ii) Secondary iii) Tertiary iv) Quaternary structures
i) Primary structure : The primary structure of protein is defined as the linear sequence of amino acid residues making up its polypeptide chain. Proteins ‘may be formed of one or more polypeptide chains. The amino acid residues are linked by peptide bonds. The peptide bond is formed between carboxyl group of one amino acid and amino group of adjacent amino acid.
Eg : fibroin of silk.
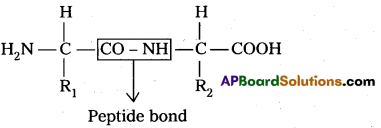
R1 – amino acid 1
R2 – amino acid 2
ii) Secondary structure : The secondary structure explains the shape of the polypeptide chain. It is derived from the primary structure by the formation of hydrogen bond interactions between amino acid residues.
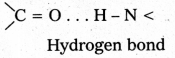
Disulfide bonds also occur either in same chain or between polypeptide chains. The secondary structure is classified into two types namely helical structure and pleated sheets.
Eg. : Keratin of hair
iii) Tertiary structure : Tertiary structure is exhibited by proteins having only one polypeptide chain. The three dimensional structure of a protein is referred to as tertiary structures. Fibrous and globular proteins are formed by tertiary structures. Various bonds are responsible for tertiary structure.
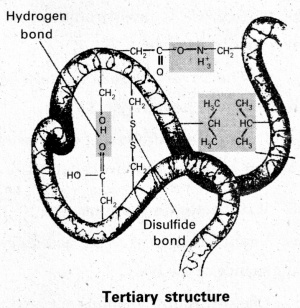
- Hydrophobic interactions
- Hydrogen bonds
- Disulphide bonds (- CH2 – S – S – CH2 -)
- Ionic or electrostatic interactions
- Vander Waal forces
e.g : Myoglobin, Ribonuclease
iv) Quaternary structure : Two or more poly- peptide chains associate together to protect quaternary structure. A protein with a quaternary structure is referred to as an oligomes.
Eg. : Hemoglobin a chain
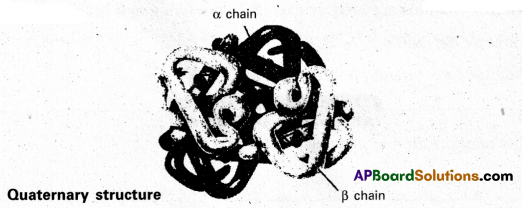
Question 8.
Explain the denaturation of proteins. [T.S. Mar. 16]
Answer:
The phenomenon of disorganization of native protein structure is known as denaturation. Denaturation results in the loss of secondary, tertiary and quaternary structure of proteins. This involves a change in physical, chemical and biological properties of protein molecules.
Agents of denaturation :
Physical Agents – Heat, violent shaking, X – rays, UV – radiation.
Chemical Agents – Acids, alkalies, organic solvents, urea, salts of heavy metals.
![]()
Question 9.
What are enzymes ? Give examples ?
Answer:
The group of complex proteinoid organic compounds, elaborated by living organism ‘which catalyse specific organic reactions are called enzymes.
Eg.: Lipases, Rennin, Maltase, Invertase etc. Practic ally all biological processes such as digestion, respiration etc., are carried on through the agency of enzymes.
Enzymes may be defined as biocatalysts synthesised by living cells.
The functional unit of enzyme is known as holo enzyme made up at apo enzyme (protein part) a co enzyme (non-protein part)

Question 10.
Explain the role of sucrose in its hydrolysis.
Answer:
- Sucrose is dextrorotatory but after hydrolysis gives dextrorotatory glucose and laevorotatory fructose.
- The laevorotation of fructose (-92.4°) is more than dextrorotation of glucose (+52.5°). This mixture is leavo rotators.
- During the hydrolysis of sucrose there is a change in the sign of rotation, from dextro (+) to laevo (-) and the product is named as invert sugar.
Question 11.
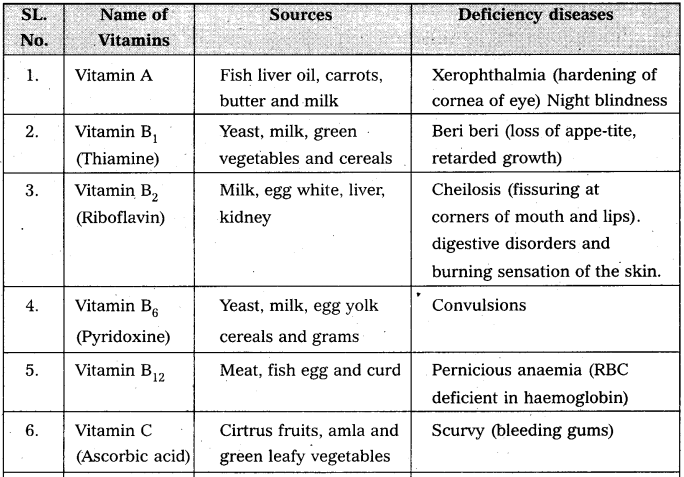
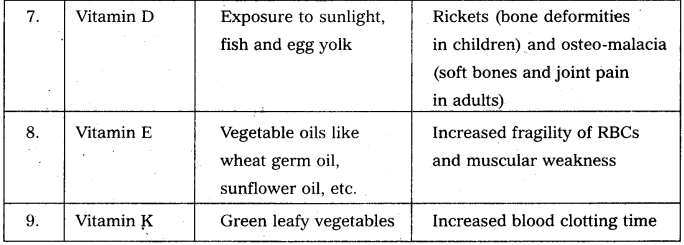
Write notes on vitamins.
Answer:
Vitamin is defined as an “accessory food factor which is essential for growth and healthy maintenance of the body.”
Classification : Vitamins are broadly classified into two major groups.
a) the fat soluble Eg : vitamin A, D, E and K.
b) water soluble Eg : vitamin B – complex and C.
Question 12.
What do you understand by “The two strands of DNA are complementary to each other” ? Explain.
Answer:
Structure of DNA : To understand the structure of DNA, we should know the charagaff s rule, which states that:
- Base composition on DNA for a particular organism is constant throughout all the somatic cells.
- Base composition always varies from one organism to another and is expressed by the dissymmetry ratio \(\frac{(A+T)}{(G+C)}\).
- Organisms closely related to each other often have similar base composition and hence – have closed values for their dissymmetry ratios.
- In a given organism the amount of adenine is always equal to the amount of thymine (A = T) and amount of guanine is equal to amount of cytosine (G = C).
- In a given organism the total amount of purine, bases
are always equal to total number of pyrimidine bases (i.e.,) (A + G = T + C).
M. Wilkins found that within the crystal there is a repeat distance of 3.4 nm and there are ten subunits per him.
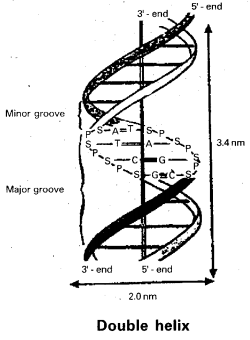
Watson – Crick model for DNA : From the above observations Watson and Crick constructed a model for DNA. This model consists of two right handed polynucleotide chains that are complimentary and coiled about the same axis to form double-helix. Some specific base pairs can be spatially accommodated and these are A – T and G – C pairs. The bases are closely associated with each other by hydrogen bonding.
The helix has diameter of about 2.0 nm and contains 10 nucleotide pairs in each turn of helix as shown above :
![]()
Question 13.
What are harmones ? Give one example for each. [A.P. & T.S. Mar. 19, 18] [Mar. 14]
i) steroid hormones
ii) Poly peptide hormones and
iii) amino acid derivatives.
Answer:
Hormones : Hormone is defined as an “organic compound synthesised by the ductless glands of the body and carried by the blood stream to another part of the body for its function”.
Eg : testosterone, estrogen.
- Example for steroid hormones : Testosterone, Estrogen
- Example for poly peptide hormones : Insulin
- Example for Amino acid derivative : Thyroidal hormones thyroxine.
Question 14.
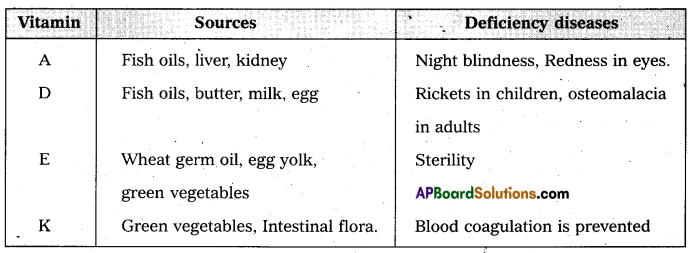
Give the sources of the following vitamins and name the diseases caused by their deficiency (a) A (b) D (c) E and (d) K [A.P. Mar. 16, 15; T.S. Mar. 15]
Answer:
Long Answer Questions
Question 1.
Explain the classification of carbohydrates.
Answer:
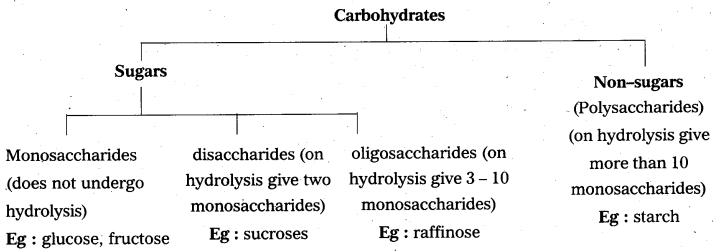
Saccharides that reduce Fehling’s reagent, Tollen’s reagent are called reducing sugars.
Eg : glucose.
Saccharides that does not reduce these reagents are called non-reducing sugars.
Eg : sucrose.
Question 2.
Discuss the structure of glucose on the basis of its chemical propeties.
Answer:
1. The molecular formula of glucose is found to be C6H12O6. It can be written as ‘CHO – (CHOH)4 – CH2OH’.
2. Acylation of glucose with acetic anhydride gives glucose pentacetate. Hence glucose molecule has five ‘-OH’ groups.
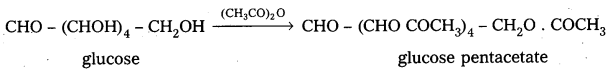
3. Glucose reacts with hydroxylamine and hydrogen cyanide forming corresponding oxime and cyano hydrin respectively. Hence glucose molecule has carbonyl group.
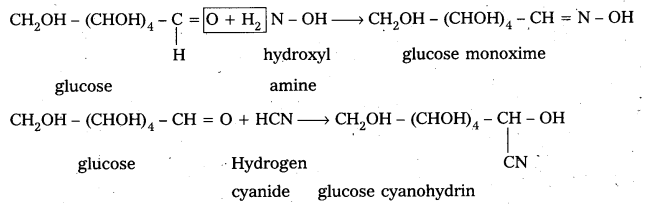
4. Glucose on oxidation with Tollen’s reagent and Fehling’s solution gives gluconic acid. Hence carbonyl group present in glucose is aldehydic group.
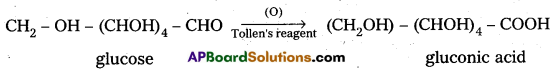
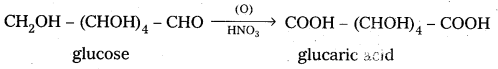
5. Glucose on oxidation with nitric acid gives glucaric’(saccharic) acid. This suggests that glucose molecule has primary alcoholic group (-CH2OH)
6. On reduction with HI, glucose gives n – hexane.
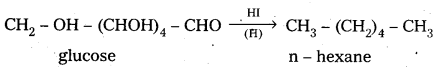
This reaction suggests that all six carbons in glucose are linearly linked.
7. Open chain structure of glucose:
Based on above observations, glucose is given the open chain structure.
8. Objections against open chain structure of glucose :
Open chain structure of glucose could not explain
- Why glucose does not respond to schiff s test.
- Why it does not react with NaHSO3 and NH3.
- Why there is mutarotation.
Mutarotation :
Change in specific rotation of a compound from certain value to fixed value is known as “Mutarotation”.
The specific rotation of α – glucose is + 110° and that of β – form is + 19.7°. If each of them is separately dissolved in water and allowed to stand, their specific rotation gradually change and reach to a specific constant value + 52.5°.
9. Cyclic structure of glucose :
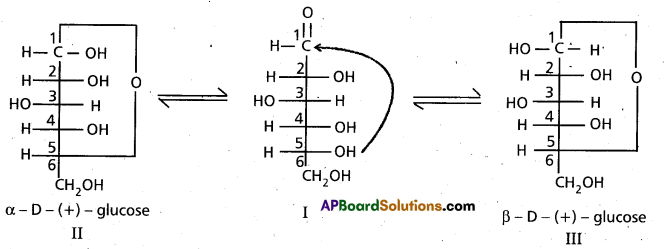
These two structures have difference in configuration at ‘C1‘ only and are called
ANOMERS.
Cyclic structure of glucose explains all the properties of glucose.
10. Pyranose structure of glucose :
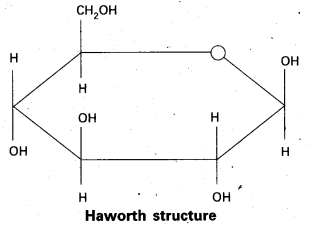
![]()
Question 2.
Write notes on
a) fructose
b) sucrose
c) maltose
d) lactose
Answer:
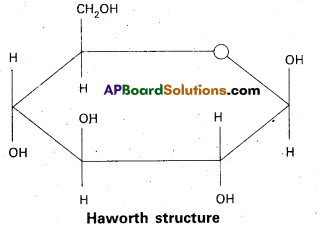
a) Fructose :
- Fructose is an important ketohexose.
- It is formed by the hydrolysis of sucrose.
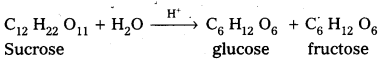
- It has the ketonic group at C – 2 and six carbons in straight chain as in the glucose.
- It has furanose ring structure.
- Open chain structures:
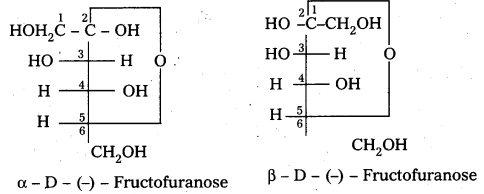
α – D (-) – Fructo furanose structure
β – D (-) – Fructo furanose structure
Haworth structures :

b) Sucrose :
Sucrose is a disaccharide. It under go hydrolysis and forms equimolar mixture of D(+) – glucose arid D(-) fructose.
C12 H22 O11 + H2O → C6 H12 O6 (glucose ) + C6 H12 O6 (fructose)
In sucrose two monosaccharide units are held together by a glycosidic linkage between C- 1 of α – glucose and C – 2 of β – glucose.
Sucrose is a non reducing sugar.
During the hydrolysis of sucrose there is a change in the sign of rotation, from dextro (+) to laevo (-) and the product is named as invert sugar.
c) Structure of maltose :
Maltose is formed from two α – glucose units (Disaccharide)
C – 1 of one glucose linked to C – 4 of another glucose
It is a reducing sugar because C – 1 of 2nd glucose is solution have free aldehyde group.
maltose on hydrolysis to form two glucose units
C12 H22 O11 (Maltose) + H2O → C6 H12 O6 (Glucose ) + C6 H12 O6 (Glucose)
d) Structure of lactose :
- Lactose is milk sugar
- Lactose is formed by the combination of β – glucose and β – galactose (Disaccharide).
- The linkage between C – 1 of galactose and c – 4 of glucose >
- It is a reducing sugar
- Lactose on hydrolysis to form glucose and galactose
C12 H22 O11 (Lactose) + H2O → C6 H12 O6 (glucose ) + C6 H12 O6 (galactose)
Question 3.
Write notes on
a) starch
b) cellulose
c) importance of carbohydrates.
Answer:
a) Starch:
Polysaccharides : The saccharides which on hydrolysis to form large number of monosaccharides are called polysaccharides.
Eg : Starch and cellulose Starch :
- Starch is the most important dietary source for human beings.
- Vegetables, roots, cereals are important sources of starch.
- It is a polymer of a – glucose.
- It is constituted by two components Amylose and amylopectin
Amylose :
- It constitutes 15 – 20% of starch -4 Anylose is water soluble component.
- Amylose is a branched chain with 200 – 1000 α – D – glucose units held by C – 1 to C – 4 glycosidic linkage.
Amylopectin :
- Amylopectin constitutes 80 – 85% of starch.
- It is a branched chain polymer of a – glucose units in which chain is formed by C – 1 to C – 4 glycosidic linkage whereas branching occurs by C – 1 to C – 6 glycosidic linkage.
b) Cellulose :
- Cellulose occurs in plants and it is the most abundant organic substance.
- It is a major constituent of cell wall of plant cells.
- Cellulose is a straight chain polysaccharide composed only of β – D – glucose units – which are joined by glycosidic linkage between C – 1 of one glucose and C – 4 of the next glucose.
c) Importance of carbohydrates :
- Carbohydrates are essential for life of plants.
- Carbohydrates are used as storage molecules as starch in plants.
- Cell wall of plants is made up of cellulose.
- Carbohydrates are also essential for life of animals. Carbohydrates are used as storage molecules as glycogen in animals.
- Carbohydrate source honey is used for a long time as an instant source of energy.
![]()
Question 4.
Write notes on amino acids.
Answer:
The organic compounds which contain amino (-NH2) functional group and carboxyl (-COOH) functional group are called amino acids.
Eg : Glycine, Alanine etc.
Essential amino acids : The amino acids that cannot be synthesised by the body and must be supplied in the diet are called essential amino acids.
Eg : valine, leucine, phenyl alanine etc.,
Non-essential amino acids : Other amino acids synthesised by the tissues of the body are called non-essential amino acids.
Eg : Glycine, alanine etc.,
Zwitter ion : In aqueous solution Of amino acids, the carboxyl group can lose a proton and amino acid can accept that proton to form a dipolar ion. This is called as zwitter ion.
Peptide linkage :
Amide 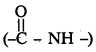 linkages between amino acids are known as peptide linkages. The product obtained from two amino acid molecules through peptide bond is called a dipeptide. Peptide chain may be extended to many amino acids.
linkages between amino acids are known as peptide linkages. The product obtained from two amino acid molecules through peptide bond is called a dipeptide. Peptide chain may be extended to many amino acids.
Proteins are naturally occufing polypeptides that contain more than 50 amino acid units.
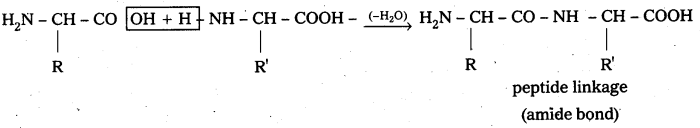
![]()
Question 5.
Write notes on proteins.
Answer:
Proteins : A poly peptide with more than hundred amino acid residues, having molecular mass higher than 10,000 units is called a protein.
g : keratin, myosin, insulin.
With respect to peptide bond proteins classified into two types.
- Fibrous proteins
- Globular proteins.
When the chains of polypeptides coil around to give a spherical shape then globular proteins are formed. These are usually soluble in water.
Eg : insulin and albumins.
When the poiy peptide chains run parallel and are held together by hydrogen and disulphide bonds then fibre – like structure is formed. These are called fibrous proteins. These are insoluble in water.
Eg: keratin, myosin.
Protein Denaturation: The phenomenon of disorganization of native protein structure is known as denaturation. Denaturation results in the loss of secondary; tertiary,. nad quaternary structure of proteins. This involves a change in physical, chemical and biological properties of protein molecules.
Agents of denaturation:
Physical agents – Heat, violent shaking, X – rays, Uy – radiation
Chemical agents – Acids, alkalies, organic solvénts, urea, salts of heavy metals.
Protein strúctures and shapes are studied at four different levels as:
i) Primary
ii) Secondary
iii) Tertiary
iv) Quaternary structures
i) Primary structure: The primary structure of protein is defined as the linear sequence of amino acid residues making up its polypeptide chain. Proteins may be formed of one or more polypeptide chains. The amino acid residues are linked by peptide bonds, The peptide bond is formed between carboxyl group of one amino açid and amino group of adjacent amino acid.
Eg: fibroin of silk.
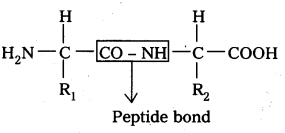
R1 – amino acid 1
R2 – amino acid 2
ii) Secondary structure : The secondary structure explains the shape of the polypeptide chain. It is derived from the primary structure by the formation of hydrogen bond interactions between amino acid residues.
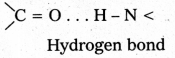
Disulfide bonds also occur either in same chain or between polypeptide chains. The secondary structure is classified into two types namely helical structure and pleated sheets.
Eg. : Keratin of hair

iii) Tertiary structure i Tertiary structure is exhibited by proteins having only one polypeptide chain. The three dimensional structure of a protein is referred to as tertiary structures. Fibrous and globular proteins are formed by tertiary structures. Various bonds are responsible for tertiary structure.

- Hydrophobic interactions
- Hydrogen bonds
- Disulphide bonds (- CH2 – S – S – CH2 -)
- Ionic or electrostatic interactions
- Vander Waal forces
e.g : Myoglobin, Ribonuclease
iv) Quaternary structure : Two or more poly- peptide chains associate together to protect quaternary structure. A protein with a quaternary structure is referred to as an oligomes.
Eg. : Hemoglobin a chain

![]()
Question 6.
Write notes on
(a) enzymes and
(b) vitamins.
Answer:
Enzymes :
The group of complex proteinoid organic compounds, elaborated by living organism which catalyse specific organic reactions are called enzymes. Eg.: Lipases, Rennin, Maltase, Invertase etc. Practically all biological processes such as digestion, respiration etc., are carried on through the agency of enzymes.
Enzymes may be defined as biocatalysts synthesised by living cells.
The functional unit of enzyme is known as holo enzyme made up at apo enzyme (protein part) and co enzyme (non-protein part)
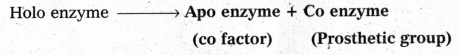
b) Vitamins :
Vitamin is defined as an “accessory food factor which is essential for growth and healthy maintenance of the body.”
Classification : Vitamins are broadly classified into two major groups.
a) the fat soluble Eg : vitamin A, D, E and K.
b) water soluble Eg : vitamin B – complex and C.


Question 7.
Explain the structures of DNA and RNA.
Answer:
Structure of DNA : To understand the structure of DNA, we should know the charagaff s rule, which states that :
- Base composition on DNA for a particular organism is constant throughout all the somatic cells.
- Base composition always varies from one organism to another and is expressed by the dissymmetry ratio \(\frac{(\mathrm{A}+\mathrm{T})}{(\mathrm{G}+\mathrm{C})}\)
- Organisms closely related to each other often have similar base composition and hence have closed values for their dissymmetry ratios.
- In a given organism the amount of adenine is always equal to the amount of thymine (A = T) and amount of guanine is equal to amount of cytosine (G = C).
- In a given organism the total amount of purine bases are always equal to total number of pyrimidine bases (i.e.,) (A + G = T + C).
M. Wilkins found that within the crystal there is a repeat distance of 3.4 nm and there are ten subunits per turn.
Watson – Crick model for DNA : From the above observations Watson and Crick constructed a model for DNA.
This model consists of two right handed polynucleotide chains that are complimentary and coiled about the same axis to form double-helix. Some specific base pairs can be spatially accommodated and these are A – T and G – C pairs. The bases are closely associated with each other by hydrogen bonding.

The helix has diameter of about 2.0 nm and contains 10 nucleotide pairs in each turn of helix as shown above :
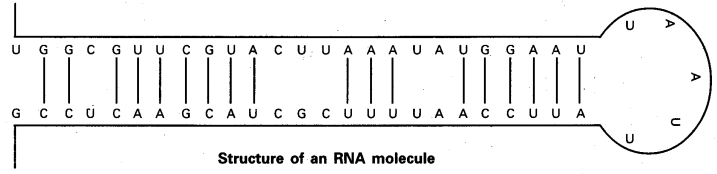
Structure of RNA: The native RNA is single stranded rather than a double stranded helical structure characteristic of DNA. However, given the complementary base sequence with opposite polarity, the single strand of RNA may fold back on itself like a hairpin and thus acquire double stranded pattern. In the region of hairpin loops, “A” pairs with ‘U’ and ‘G’ pairs with ‘C’. The base pairing in RNA hairpins is frequently imperfect. The proportion of helical regions in various types of RNA varies over a wide range.
![]()
Question 8.
Write notes on the functions of different hormones in the body.
Answer:
Functions of Hormones :
- Hormones help to maintain the balance of biological activities in the body.
- Insulin maintains the blood glucose level with in the limit.
- Growth hormones and sex hormones play role in growth and development.
- Low level of thyroxine (produced from thyroid gland) causes hypothyroidism. High level of thyroxine causes hyper thyroidism. .
- gluco corticoids control the carbohydrate metabolism, modulates the inflammatory reactions.
- The mineralo corticoids control the level of excretion of water and salt by the kidney.
- Adrenal cortex does not function properly then results in Addison’s disease.
- Hormones released by gonads are responsible for development of secondary sex characters.
- Testosterone is responsible for development of secondary sex hormone produced in male.
- Estradiol is the main female sex hormone responsible for development of secondary female characterstics like control of menustrual cycle.
- Progesterone is responsible for preparing the uterus for implantation of fertilised egg.
Intext Questions
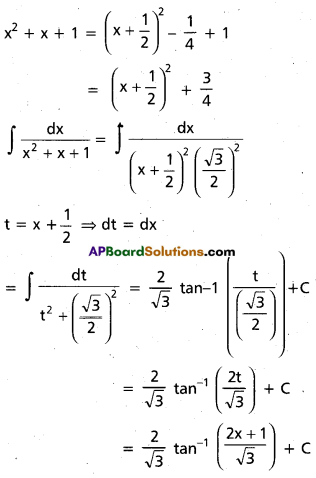
Question 1.
Glucose or sucrose are soluble in water but cyclohexane and benzene (simple six membered ring compounds) are insoluble in water. Explain.
Answer:
In glucose (C6H12O6), 5 – OH groups are present and in sucrose (C12H22O11), B – OH groups are present, which are polar in nature. They are involved in intermolecular hydrogen bonding with water molecules, so these compounds are readily soluble in water.
Benzene (C6H6) and cyclohexame (C6H12) are hydrocarbons. They do not have any polar group so they cannot make hydrogen bonds with water. Therefore, they are insoluble in water.
Question 2.
What are the expected products of hydrolysis of lactose ?
Answer:
Lactose forms two molecules of monosaccharides on hydrolysis – One molecule of D-(+) – glucose and one molecule of D – (+) – galactose.
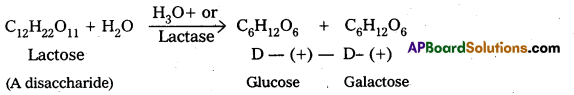
Question 3.
How do you explain the formation of furanose structure for fructose while glucose forms pyranose structure with same molecular formula C6H12O6 ?
Answer:
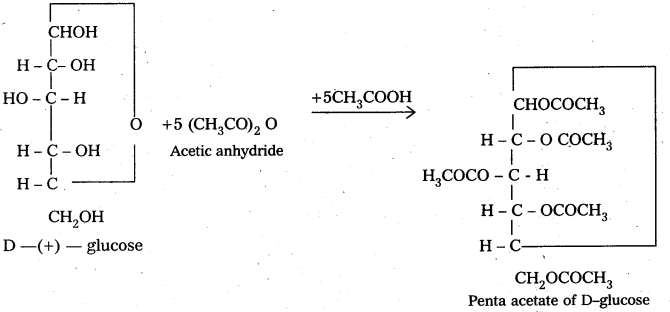
D-glucose, an aldohexose given the characteristic reactions of aldehydic group (e.g., with HCN Tollen’s reagent, Fehling reagent etc.,) but penta acetae of D-glucose does not give these reactions. It means that either – CHO group is absent or it is not available for the chemical reactions in penta acetyl glucose. Infact, the aldehydic group is a part of hemiacetal structure which penta acetyl glucose has. So, it is not available for reaction.
![]()
Question 4.
The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
Higher the polarity of a group, more is its solubility in water.
Answer:
Amino acids are dipolar in nature (N+ H3 – CHR – COO–) and have strong dipolar interatctions.
Therefore, these have high melting points. When dissolved in water, these form hydrogen bonds with water molecules, therefore, these are soluble in water, (except at isoelectric point, where they have least solubility) In case of halo acids, these are not dipolar. Only their carboxyl group is involved in hydrogen bonding, not the halogen atom. Therefore, halo acids, have lower melting points and lesser solubility than amino acids.
Question 5.
Why cannot vitamin C be stored in our body ?
Answer:
VitaminC (ascorbic acid) is water soluble. Therefore, it is regularly excreted in urine from the body and cannot be stored.
Question 6.
What products would be formed when a nucleotide from DNA containing thymine is hydrolysed ?
Give the composition of DNA molecule as on hydrolysis it gives its all constituents.
Answer:
When a nucleotide from DNA is hydrolysed, it will give deoxyribose sugar molecules, phosphoric acid, pyrimidine bases, i.e., guanine (G) and adenine (A) and purine bases, i.e., thymine (T) and cytosine (C).
![]()
Question 7.
When RNA is hydrolysed, there is no relationship among the quantities of different bases obtained. What does this fact suggest about the structure of RNA ?
Single stranded structure of RNA.
Answer:
DNA molecule has a double strand structure and four complementary bases paired with each other. Cytosine (C) pairs with guanine (G) while thymine (T) pairs with adenine (A). Due to such structure, DNA produces the products in definite molar ratio.
The case is not same in RNA, ie., base pairing rule is not followed. Thus, when RNA is hydrolysed, the quantities of different bases are different. This suggests that RNA has single stranded structure.
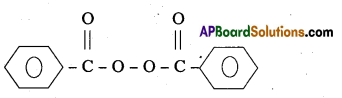
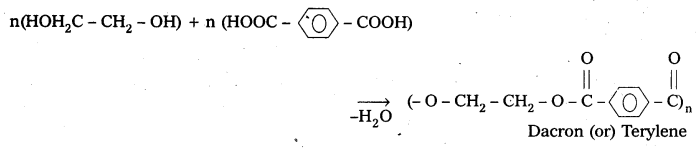
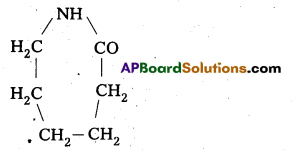
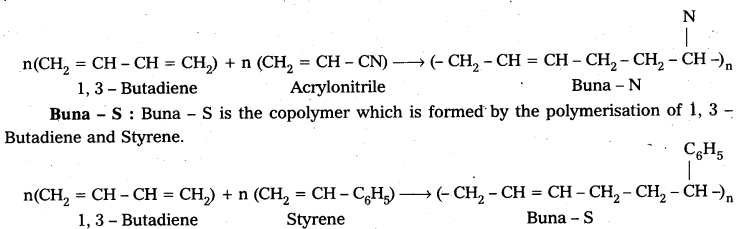
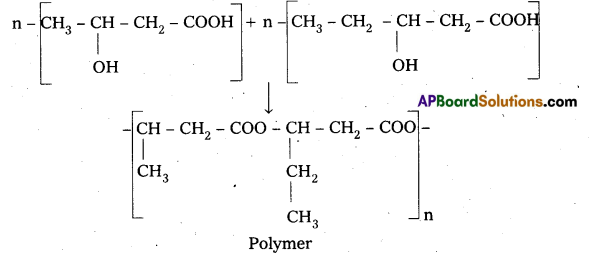
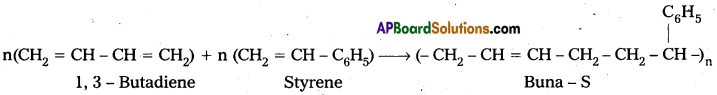
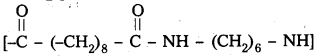 are decane dioic acid HOOC -CH2-)8 COOH and hexamethylene diamine H2N – (CH2-)6 NH2
are decane dioic acid HOOC -CH2-)8 COOH and hexamethylene diamine H2N – (CH2-)6 NH2