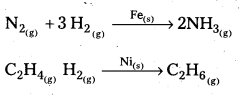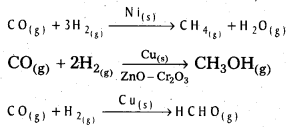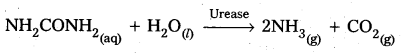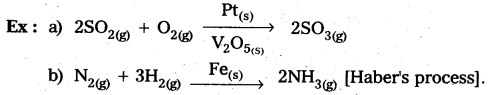Andhra Pradesh BIEAP AP Inter 2nd Year Chemistry Study Material 5th Lesson General Principles of Metallurgy Textbook Questions and Answers.
AP Inter 2nd Year Chemistry Study Material 5th Lesson General Principles of Metallurgy
Very Short Answer Questions
Question 1.
What is the role of depressant in froth floatation?
Answer:
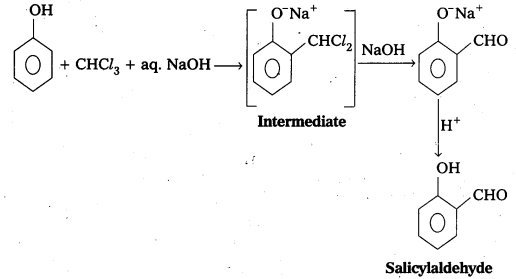
By using depressants in the froth floatation process, it is possible to separate a mixture of two sulphide ores.
Eg: In the ore containing ZnS and PbS, the depressant used is NaCN. It prevents ZnS from coming to the froth but allows PbS to come with the froth.
Question 2.
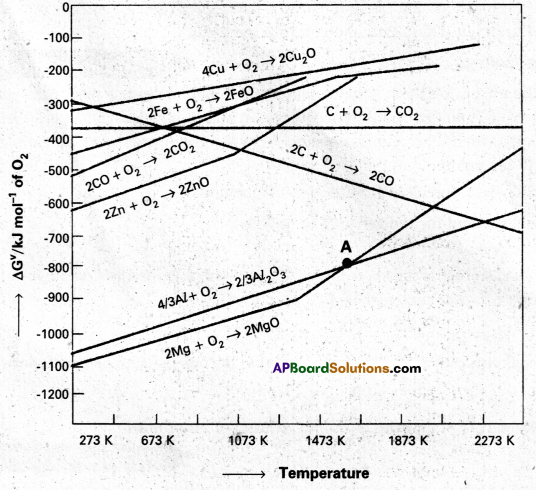
Between C and CO, which is a better reducing agent at 673K.
Answer:
- Out of C and CO, Carbon nonoxide (CO) is a better reducting agent at 673K.
- At 983K (or) above coke(C) is better reducing agent.
- The above observations are form Ellingham diagram.
![]()
Question 3.
Name the common elements present in the anode mud in the eletrolytic refining of copper.
Answer:
- The common elements present in the anode mud in eletrolytic refining of copper are less reactive valuable metals, silver (Ag), gold (Au) and platinum (Pt) etc….
- These elements donot lose eletrons at anode and collect under the anode as anode mud.
Question 4.
State the role of silica in the metallurgy of copper.
Answer:
The role of silica in the metallurgy of copper is to àcts as an acidic flux. Silica reacts with the impurities of iron and form slag.
FeO (Gangue) + SiO2 (flux) → FeSiO3 (Slag)
Question 5.
Explain “poling. [A.P. Mar.16, 15]
Answer:
When the metals are having the metal oxides as impurities this method is employed. The impure metal is melted and is then covered by carbon powder. Then it is stirred with green wood poles. The reducing gases formed from the green wood and the carbon, reduce the oxides to the metal.
Eg: Cu & Sn metals are refined by this method.
Question 6.
Decribe a method for the refining of nickel.
Answer:
Mond’s process:
- In Mond’s process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetra carbonyl.
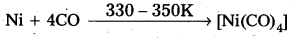
- Nickel tetra carbornyl is strongly heated to decompose and gives the pure Nickel
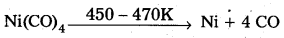
![]()
Question 7.
How is cast iron different from pig iron ?
Answer:
- Cast iron is formed by melting pig iron with scrap iron and coke in presence of blast of hot air. It contains carbon content 3%(approximately). It is extremely hard and brittle.
- Pig iron is formed from blast furnace. It contains carbon 4% (approximately). It contains small quantities of impurities S, P, Si, Mu etc….
Question 8.
What is the difference between a mineral and an ore ?
Answer:
Minerals : The naturally occuring chemical compounds of metal in the earth’s crust are called minerals.
Ore : The mineral from which metal can be extracted economically is called ore.
Question 9.
Why copper matte is put in silica lined converter ?
Answer:
Copper matte conains Cu2 sand FeS. In this mixture FeS is gangue. For removing the gangue, silica present in the lining of the Bessemer’s converter acts as acidic flux and forms slag.
2FeS (gangue) + 3O2 → 2FeO + 2SO2
FeO (gangue) + SiO2 (flux) → FeSiO3 (Slag)
Question 10.
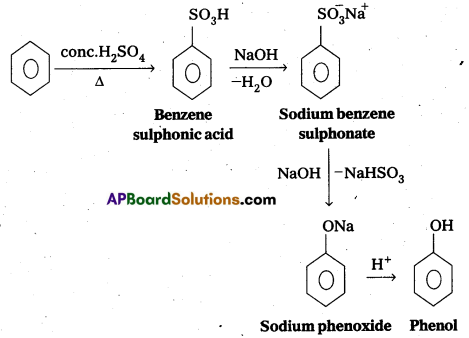
What is the role of cryolite in the metallurgy of aluminium? [T.S. Mar. 16, 15]
Answer:
By adding the cryolite to the pure Alumina, the melting point of pure Alumina is lowered (which is very high 2324K) and eletrical conductivity of pure alumina is increased.
Question 11.
How is leaching carried out in the case of low grade copper ores?
Answer:
In case of low grade ores of copper, hydrometallurgy technique is used for extraction. Here leaching process can be done by using acids (or) bacteria, The solution containing Cu+2 is treated with scrap iron (or) H2.
Cu+2(aq) + H2(g) → Cu(S) + 2H+(aq)
![]()
Question 12.
Why is zinc not extracted from zinc oxide through reduction using CO?.
Answer:
Zinc is not extracted from zinc oxide through reduction by using CO.
Explanation:
2Zn + O2 → 2ZnO, ∆G° = -650 IcI
2C0 + O2 → 2CO, ∆G° = -450 kJ
2ZnO + 2CO → 2Zn, 2CO2, ∆G° = 200 kJ
∆G° = Positive indicatis that the reaction is not feasible.
Question 13.
Give the composition of the following alloys. [A.P. & T.S. Mar. 19, 17] [Mar. 14]
a) Brass
b) Bronze
c) German Silver
Answa:
a) Composition of Brass : 60 – 80% Cu, 20 – 40% Zn .
b) Composition of Bronze : 75 – 90% Cu, 10 – 25% Sn
c) Composition of German silver : 50 – 60% Cu, 10 – 30% Ni, 20 – 30% Zn.
Question 14.
Explain the terhts gangue and slag.
Answer:
Gangue : The ore is contaminated with the minerals of earth crust and undesired chemical compounds. These are known as gangue (or) matrix.
Slag: The fused material obtained during the refining (or) smelting of metals by combining the flux with gangue is called slag.
Eg : FeO (gangue) + SiO2 (flux) → FeSiO3 (Slag)
Question 15.
How is Ag or Au obtained by leaching from the respective ores ?
Answer:
Ag and Au are leached with a dilute solution of NaCN (or) KCN in presence of 02(air). From the leached solution the metal is obtained through displacement by Zinc. .
4M(s) + 8eN–(aq) + 2H2O(aq) + O2(g) → 4[M(CN)2]–(aq) + 4OH–(aq)
2[M(CN)2 ]–(aq) + Zn(s) → [Zn(CN)4]2-(aq) + 2M(s)
Question 16.
What are the limitations of Ellingham diagram ?
Answer:
Limitations of Ellingham diagram :
- Ellingham diagram is based only on the thermodynamic concepts. It does not explain the kinetics of the reduction process. The graph simply indicates whether a reacton is possible or not but not the kinetics of the reaction.
- The interpretation of ∆G° is depends on K [ ∆G° = -RTl.nK]
It is presumed that the reactants and products are in equilibrium. .
MxO + Ared ⇌ xM + AOox
This is not always true because the reactant or product may be solid.
![]()
Question 17.
Write any two ores with formulae of the following metals :
(a) Aluminium
(b) Zinc
(c) Iron
(d) copper
Answer:
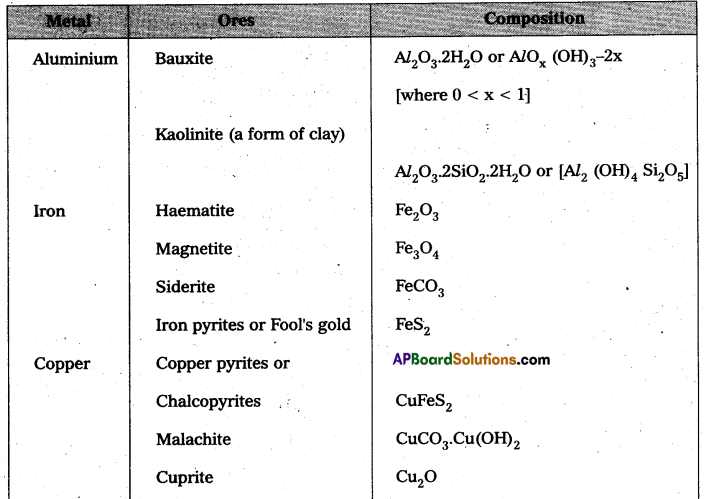
a) Ores of Aluminium : Bauxite – Al2 O3. 2H2O
Cryolite – Na3AlF6
b) Ores of Zinc : Zinc blende – Zns
Calamine – ZnCO3
c) Ores of Iron : Haematite – Fe2O3
Magnetite – Fe3O4
d) Ores of copper : Copper pyrites – CuFeS2
Copper glance – Cu2S.
Question 18.
What is matte ? Give its composition.
Answer:
During the extraction of ‘Cu’ from copper pyrites the product of the blast furnace consists mostly of Cu2S and a little of FeS. This product is known as “Matte”. It is collected from the outlet at the bottom of the fumance.
Question 19.
What is blister copper ? Why is it so called ? [T.S. Mar. 18]
Answer:
During the extraction of ‘Cu’ from copper pyrites when the matte is charged into a Bessemer converter then cuprous oxide combine with cuprous sulphide and forms Cu metal.
2CU2O + Cu2S → 6Cu + SO2
The ‘Cu’ meted is cooled.
The obtained copper metal is impure and is known as “Blister copper” (98% pure).
The solid fied copper has blistered appearance due to evolution of SO2 Hence it is called blister copper.
Question 20.
Explain magnetic separation of impurities from an ore.
Answer:
Electro-magnetic method : The method is used if the gangue or the ore particles are magnetic in nature. The finely powdered ore is dropped on a belt moving on two strong electromagnetic rollers. The magnetic and the non-magnetic substances form two separate heaps.
For example, let the ore particles be magnetic and then the non-magnetic material will be the gangue. The non-magnetic material forms a heap nearer to the magnetic roller.
Eg : Haematite and magnetite hae magnetic ore particles. Cassiterite or Tin stone has Wolframite as magnetic impurity.
Question 21.
What is flux ? Give an example.
Answer:
Flux : An outside substance added to ore to lower its melting point is known as flux.
- Flux combines with gangue and forms easily fusible slag.
gangue + flux → slag
Eg: FeO (gangue) + SiO2 (flux) → FeSiO3 (Slag
![]()
Question 22.
Give two uses each of the following metals :
(a) Zinc
(b) Copper
(c) Iron
(d) Aluminium
Answer:
a) Uses of Zinc :
- Zinc is used in large quantities in batteries
- Zinc is used for galvanising iron.
- Zinc is used in manufacturing alloys.
Eg: Brass, German silver,
b) Uses of copper :
- Copper is used in manufacturing of water pipes and steam pipes.
- Copper is used in making wires used in electrical industry.
- Copper is used in manufacturing of alloys.
Eg : Brass, Bronze. .
c) Uses of Iron:
- Cast iron is used in casting stoves, railway siéepers- gutter pipers, toys etc.
- It is used in manufacturing of wrought iron and steel.
- Wrought iron is used in making anchors, wires, bolts, chains etc.
d) Uses of Aluminium :
- Aluminium foils are used as wrappers for chacolates.
- Aluminium is fine dust used in paints and lacquers.
- Aluminium is used in photo frames.
- Aluminium is used in extraction of Cr and Mn from their oxides.
Question 23.
Between C and CO, which is a better reducing agent for ZnO ?.
Answer:
Case – I : [Coke as reducing agent]
ZnO + C → Zn + CO, ∆G° become lesser as the T is more then 1120K
Case – II : [CO as reducing agent]
ZnO + CO2 → Zn + CO, ∆G° becomes lesser when the T is more then 1323K.
- The value of ∆G° is negative for a reaction to occur.
- In the equation (1) ∆G° becomes negative at low temperature- so equation (1) is feasible i. r. C is a better reducing agent for ZnO.
Question 24.
Give the uses of
a) Cast iron
b) Wrought iron
c) Nickel steel
d) Stainless steel
Answer:
a) Uses of Cast iron :
- Cast iron is used for casting stoves, railway sleepers, gutter pipes, toys etc.
- It is used in manufacturing of wrought iron and steel.
b) Uses of wrought iron :
- Wrought iron is used in making anchors, wires.
- Wrought iron is used in making chains and agricultural implements.
c) Uses of Nickel steel:
- Nickel steel is used for making cables, automobiles and aeroplane parts.
- Nickel steel is used for making pendulum, measuring tapes, chrome steel for cutting tools and crashing machines.
d) Uses of stainless steel:
- Stainless steel is used in manufacturing of cycles, automobiles.
- Stainless steel is used in manufacturing of utensils, pens etc.
![]()
Question 25.
How is aluminium useful in the extraction of chromium and manganese from their oxides ?
Answer:
- ’AT’ is used as reducing agent.
- 4 By Alumino thermite process Cr, Mn are extracted from their oxides.
- The reactions are highly exothermic.
Cr2O3 + 2Al → 2Cr + Al2O3
3Mn3O4 + 8Al → 4Al2O3 + 9Mn
Short Answer Questions
Question 1.
Copper can be extracted by hydrometallurgy but not zinc – explain.
Answer:
Copper can be extracted by hydromefallurgy but not zinc.
Explanation:
- E0 Value of Zn+2/Zn = -0.762V is less than that of E0 value of Cu+2/Cu = 0.337V .
- From the above data zinc is a stronger reducing agent and can easily displace the Cu+2 ions present in the complex.
[Cu(CN)2] + Zn (Soluble complex → [Zn(CN)2] + Cu (Precipitate) - Zinc can be isolated by hydrometallurgy only when stronger reducing agents than zinc are present.
- So zinc cannot be extracted by hydrometallurgy.
Question 2.
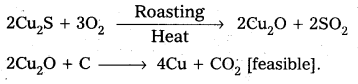
Why is the extraction of copper form pyrites more difficult then that from its oxide ore through reduction?
Answer:
The extraction of copper from pyrites is more difficult than that from its oxide ore through reduction.
Explanation:
Pyrites (Cu2S) Cannot be reduced by carbon(or) hydrogent because the standard free energy of formation (∆G°) of Cu2S is greater then those of CS2 and H2S.
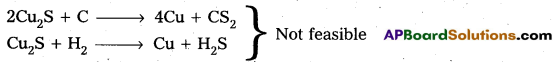
The ∆G° of copper oxide is less than that of CO2.
∴ The sulphide ore is first converted to oxide by roasting and then reduced.
Question 3.
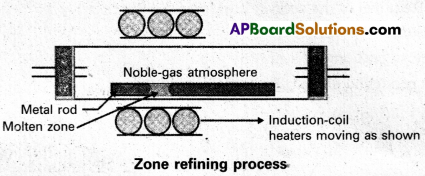
Explain zone refining.
Answer:
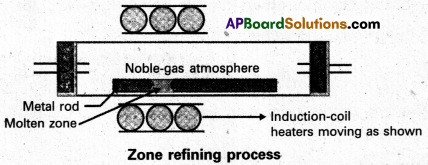
Zone refining:
- Zone refining is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal.
- A circular mobile heater is fixed at one end of a rod of impure metal.
- The molten zone moves along with the heater moves forward the pure metal crystallises out of the melt and the impurities pass into the adjacent molten zone.
- The above process is repeated several times and the heater is moved in the same direction form one end to the other end. At one end impurities get concentrated. This end is cut off.
- This method is very useful for producing semiconductor grade metals of very high purity. Eg : Ge, Si, B, Ga etc…
Question 4.
Write down the chemical reactions taking place in the extraction of zinc from zinc blende.
Answer:
In the extraction of zinc from zinc blende the following chemical reactions taking place.
i) Roasting : The process of strong heating the zinc blende in presence of air is roasting.
![]()
ii) Reduction : Zinc oxide obtained by roasting undergo reduction with coke.
![]()
![]()
Question 5.
Write down the chemical reactions taking place in different zones in the blast furnace during the extraction of iron.
Answer:
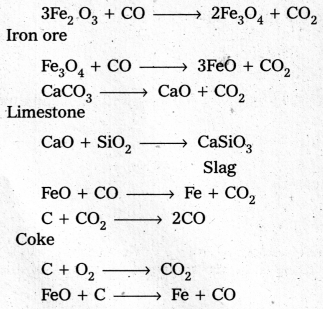
The chemical reactions taking place in different zones in the blast furnace during the extraction of iron are
Question 6.
How is alumina separated from silica in the bauxite ore associated with silica ? Give equations.
Answer:
Serpeck’s process is used for the purification of white Bauxite i.e., Bauxite containing silica as impurity.
In this process bauxite is mixed with coke and is then heated to 2075K in a current of nitrogen. Silica is reduced to silicon and escapes as a vapour.
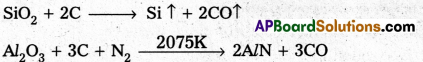
Aluminium nitride formed in the above step is hydrolysed to form aluminium hydroxide.
AlN + 3H2O → AZ(OH)3 ↓ + NH3 ↑
Al(OH)3 PPt is washed, dried and then ignited to from purified bauxite.
![]()
Question 7.
Giving examples to differentiate roasting and calcination. [T.S. Mar. 19, 18, 16, 15; A.P. Mar. 16, 15]
Answer:
Roasting: Removal of the volatile components of a mineral by heating mineral either alone (or) mixed with some other substances to a high temperature in the presence of air is called Roasting.
- It is applied to the sulphide ores.
- SO2 gas is producted along with metal oxide.
Eg : 2ZnS + 3O2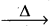 2ZnO + 2SO2 ↑
2ZnO + 2SO2 ↑
Calcination: Removal of the volatile components of a mineral by heating in the absence of air is called calcination.
- It is applied to carbonates and bicarbonates.
- CO2 gas is produced along with metal oxide.
Eg: CaCO3 CaO + CO2
CaO + CO2
ZnCO3 ZnO + CO2
ZnO + CO2
![]()
Question 8.
The Value of ∆G° for the formation of Cr2O3 is – 540kJ mol-1 and that of Al2O3 is – 827kJ mol-1. Is the reduction of Cr2O3 Possible with Al?
Answer:
From the given data the following are the thermo chemical equations
\(\frac{4}{3}\) Cr(s) + O2(g) → \(\frac{2}{3}\) Cr2O2(s), ∆G° – 540 KJ ……………….. (1)
\(\frac{4}{3}\) Al(s) + O2(g) → \(\frac{2}{3}\) Al2O3(s), ∆G° – 827 KJ ……………….. (2)
Equation (1) – Equation (2)
\(\frac{2}{3}\) Cr2O3(s) + \(\frac{4}{3}\) Al(s) → \(\frac{2}{3}\) Al2O3(s) + \(\frac{4}{3}\) Cr(s), ∆G° – 287 KJ
∆G° = -ve, So the reaction is feasible
Hence the reduction of Cr2O3 is possible with ‘Al’.
Question 9.
What is the role of graphite rod in the electrometallurgy of aluminium ? [A.P. Mar. 16]
Answer:
- In the electrolytic reduction of alumina by Hall – Heroult process graphite rod acts as anode.
- At anode, O2 gas is liberated which reacts with the carbon of anode to form CO2 gas. So these graphite rods are consumed slowly and need to be replaced from time to time.
Al2O3 ⇌ 2Al+3 + 3O-2
Cathode : Al+3 + 3e– → Al
Anode : 3O-2 → 3[O] + 6e–
C(s) + O2(g) → CO2(g)
Question 10.
Outline the principles of refining of metals by the following methods.
(a) Zone refining
(b) Electrolytic refining
(c) Poling
(d) Vapour phase refining.
Answer:
a) Zone refining :
- Zone refining is based on the principle that the impurities are more soluble in the melt than in the solid state of the metal. .
- A circular mobile heater is fixed at one end of a rod of impure metal.
- The molten zone moves along with the heater moves forward the pure metal crystallises out of the melt and the impurities pass into the adjacent molten zone.
- The above process is repeated several times and the heater is moved in the same direction form one end to the other end. At one end impurities get concentrated. This end is cut off.
- This method is very useful for producing semiconductor grade metals of very high purity.
Eg : Ge, Si, B, Ga etc..
b) Electrolytic refining : This process is used for less reactive metals like Cu, Ag, AZ, Au etc.
- In this process anode is made by impure metal and a thin strip of pure metal acts as cathode. .
- On electrolysis metal dissolves from anode and pure metal gets deposited at cathode.
M → Mn+ + ne–
Impure
Mn+ + ne– → M (Cathode) Pure metal
Impurities settle down below anode in the form of anode mud.
c) Poling : When the metals are having the metal oxides as impurities this method is employed. The impure metal is melted and is then covered by carbon powder. Then it is stirred with green wood poles. The reducing gases formed from the green wood and the carbon, reduce the oxides to the metal.
Eg : Cu & Sh metals are refined by this method.
d) Vapour phase refining: In this method the metal is converted into its volatile compound and collected. It is then decomposed to give pure metal. .
- The metal should form a volatile compound with an available reagent.
- The volatile compound should be easily decomposable. So the the recovery is easy.
E.g : Mond’s process : - In Mond’s process, nickel is heated in a stream of carbon monoxide forming a volatile complex, nickel tetra carbonyl.
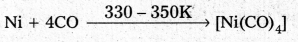
- Nickel tetra carbomyl is strongly heated to decompose and gives the pure Nickel
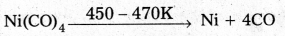
![]()
Question 11.
Predict the conditions under which Al might be expected to reduce MgO.
Answer:
The Equations for the formation of two oxides are
\(\frac{4}{3}\)Al(S) + O2(g) → 2Mg(S) + \(\frac{2}{3}\) O2(g) \(\frac{4}{3}\) 2MgO(S)
From the Ellingham diagram the two curves of these oxides formation intersect each otherat a certain point. The corresponding value of ∆G° becomes zero for the reduction of MgO by aluminium metal,
2MgO(S) + \(\frac{4}{3}\) Al(S) ⇌ 2Mg(S) + \(\frac{2}{3}\) Al2O3(S)
- From the above information the reduction of MgO by Al metal cannot occur below this temperature (1665 K) instead, Mg can reduce Al2O3 to Al below 1665 K.
- Al-Metal can reduce MgO to Mg above 1665K because ∆G° for Al2O3 is less compared to that of MgO.
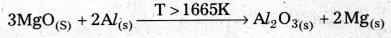
Question 12.
Explain the purification of sulphide ore by froth floatation method. [A.P. Mar.’19, 17, 15; T.S. Mar. 15]
Answer:
Froth floatation method :
This method is used to concentrate sulphide ores.
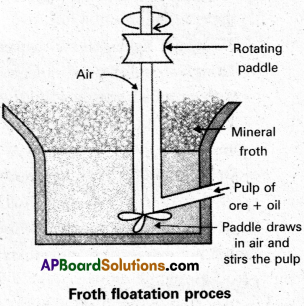
- In this process a suspension of the powdered ore is made with water.
- A rotating paddle is used to agitate the suspension and air is blown into the suspension in presence of an oil.
- Froth is formed as a result of blown of air, which carries the mineral particles.
- To the above slurry froth collectors and stabilizers are added.
- Collectors like pine oil enhance non-wettability of the mineral particles.
- Froth stabilizers like cresol stabilize the froth.
- The mineral particles wet by oil and gangue particles wet by water.
- The broth is light and is skimmed off. The ore particles are then obtained from the froth. By using depressants in froth floatation process, it is possible to separate a mixture of two suplhide ores.
Eg : In the ore containing ZnS and PbS, the depressant used is NaCN. It prevents ZnS from coming to the froth but allows PbS to come with the froth.
Question 13.
Explain the process of leaching of alumina from bauxite. [T.S. Mar. 17]
Answer:
The principal ore of aluminium, bauxite, usually contains SiO2, iron oxides and titanium oxide (TiO2) as impurities, concentration is carried out by digesting the powdered ore with a concentrated solution of NaOH at 473 – 523 K and 35 – 36 bar pressure. The way. Al2O3 is leached out as sodium aluminate (and SiO2 too as sodium silicate) leaving the other impurities behind :
Al2O3 (S) + 2NaOH(aq) + 3H2O(l) → 2 Na[Al(OH)4](aq)
The aluminate is alkaline in nature and is neutralised by passing CO2 gas and hydrated Al2O3 is precipitated. At this stage, the solution is seeded with freshly prepared samples of hydrated Al2O3 which induces the precipitation of Al2O3 xH2O
2 Na[Al(OH)4](aq) + CO2(g) → Al2O3 xH2O(s) + 2NaHCO3(aq)
The sodium silicate remains in the solution and the insoluble hydrated alumina is filtered, dried and heated to give pure Al2O3:
![]()
![]()
Question 14.
What is Ellingham diagram ? What information can be known from this in the reduction of oxides ?
Answer:
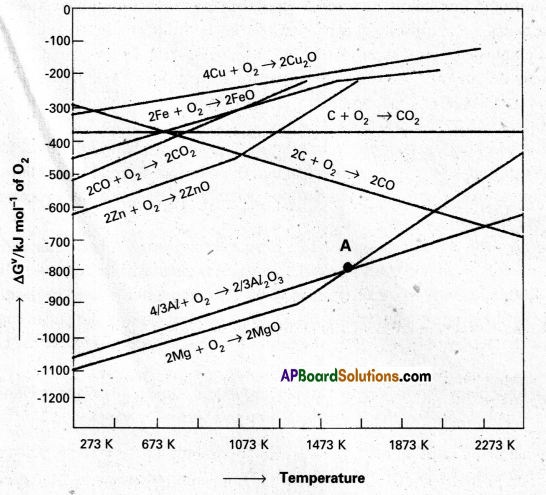
The graphical representation of Gibbs energy which provides a sound basis for considering the choice of reducing agent in the reduction of oxides. This graphical representation is known as Ellingham diagram.
- This diagram helps us in predicting the feasibility of thermal reduction of an ore.
- The Criterion of feasibility of a reaction is that at a given temperature, Gibbs energy of the reaction must be negative.
- Ellingham diagram normally consists plots of ∆G° vs T for formation of oxides of elements.
- The graph indicates whether a reaction is possible or not, i.e., the tendency of reduction with a reducing agent is indicated with a reducing agent is indicated.
- The reducing agent forms its oxide when the metal oxide is reduced. The role of reducing agent is to make the sum of ∆G° values of the two reactions negative.
- Out of C and CO, Carbon monoxide (CO) is a better reducting agent at 673K.
- At 983K (or) above coke(C) is better reducing agent.
- The above observations are form Ellingham diagram.
Zinc is not extracted from zinc oxide through reduction by using CO.
Explanation :
- 2Zn + O2 → 2ZnO, ∆G° =-650 kJ
- 2CO + O2 → 2CO2, ∆G° = -450 kJ
- 2ZnO + 2CO → 2Zn, 2CO2, ∆G° = 200 kJ
∆G° = Positive indicatis that the reaction is not feasible.
The above fact is explained on the basis of Ellingham diagram.
Question 15.
How is copper extracted from copper pyrites?
Answer:
Extraction of copper from copper pyrites :
Copper pyrite is the main source of copper metal. Various steps involved in the extraction of copper discussed below.
Step – I:
Concentration of ore by froth floatation process :
The ore is first crushed in ball mills. The finely divided ore is suspended in water. A little pine oil is added and the mixture is vigorously agitated by a current of air. The froth formed carries the ore particles almost completely. The gangue sinks to the bottom of the tank. The froth is separated and about 95% concentrated ore is obtained.
Step -II:
Roasting : To remove the volatile impurities like As (or) Sb, the ore is roasted in a free supply of air. A mixture of sulphides of copper and iron are obtained and these are partially oxidised to respective oxides.
Cu2S. Fe2S3 + O2 → Cu2S + 2FeS + SO2
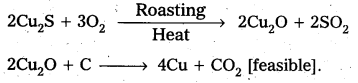
2Cu2S + 3O2 → 2Cu2O + 2SO2
2FeS + 3O2 → 2FeO + 2SO2
Step -III:
The roasted ore is mixed with a little coke and sand (Silica) and smelted in a blast furnace and fused. A blast of air, necessary for the combustion of coke, is blown through the tuyeres present at the base of the furnace. The oxidation of the sulphides of copper and iron will be completed further. A slag of iron silicate is formed according to the reactions given below :
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → Fe SiO3 Ferrous Silicate (Slag)
Cu2O + FeS → Cu2S + FeO
Step -IV : After smelting the copper ore in blast furnace, the product of the blast furnace consists mostly of Cu2S and a little of ferrous sulphide. This product is known as “Matte.” It is collected from the outlet at the bottom of the furnace. After then the following processes are carried out for getting the pure copper.
Bessemerization : The matte is charged into a Bessemer converter. A bessemer converter is a pear-shaped furnace. It is made of steel plates. The furnace is given a basic lining with lime or magnesium oxide (obtained from dolomite or magnesite). The converter is held in position by trunnions and can be tilted in any position. A hot blast of air and sand is blown through the tuyeres present near the bottom. Molten metal, the product in the furnace, collects at the bottom of the converter.
Reactions that took place in blast furnace go to completion. Almost all of iron is eliminated as a slag. Cuprous oxide combines with cuprous sulphide and forms Cu metal.
2Cu2O + Cu2S → 6Cu + SO2
The molten metal is cooled in sand moulds. S02 escapes. The impure copper metal is known as “Blister copper” and is about 98% pure.
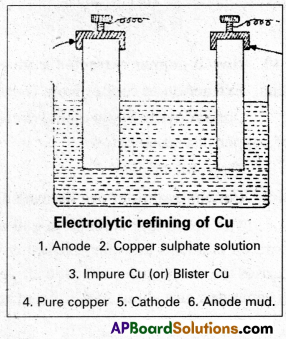
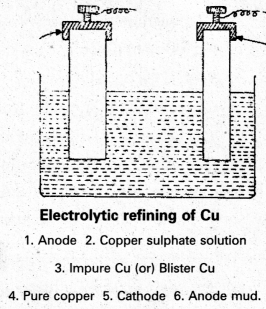
Step -V:
Refining : The Blister copper is purified by electrolysis. The impure copper metal is made into plates. They are suspended into lead – lined tanks containing Copper (II) Sulphate solution. Thin plates of pure copper serve as cathode. The cathode plates are coated with graphite. On electrolysis, pure copper is deposited at the cathode. The copper obtained is almost 100% pure Cu.
![]()
Question 16.
Explain the extraction of Zinc form Zinc blende.
Answer:
“Zinc blende (ZnS)” is an important source for ‘Zn’ metal.
Extraction of Zinc : Zinc blende ore is treated in the following stages.
i) Crushing : The ore is crushed to a fine powder in ball mills.
ii) Concentration of the ore : The ore is concentrated first by gravity process. The crushed ore is washed with a stream of water on a Wilfley’s table. The tables have a corrugated top and are under a rocking motion. Due to this motion, the lighter gangue particles are washed away by the steam. The heavier ore particles settle to the bottom of the table.
The partially concentrated ore is further concentrated by froth floatation process. The ore particles go with the froth.
The concentrated ore is subjected further to electromagnetic separation, if iron oxide is present in the gangue. Iron oxide is magnetic and so forms a heap nearer to the magnetic pole.
iii) Roasting: The concentrated ore thus obtained is roasted in rotary shelf burner which is provided with horizontal shelves and raking arms. The ore is added at the top and zinc oxide is collected from the bottom. The following reactions take place.
2ZnS + 3O2 → 2ZnO + 2SO2
ZnS + 2O2 → ZnSO4
2ZnSO4 → 2ZnO + 2SO2 + O2.
iv) Reduction : Three methods are in practice for the reduction of the oxide to the metal., The most commonly used method is the Belgian process. In this process the roasted ore is mixed with coal or coke intimately and is taken in fireclay or earthem ware retorts. The retorts are made of fireclay which are bottle shaped tubes. These are closed at one end. The other end is connected to air cooled earthem ware condenser. A large number of retorts are placed in tiers in a large furnace and heated in 1100°C by burning the gas. ‘Prolongs’ made of sheet iron are attached to the condensers. The metal condenses in these earthem ware condensers and the prolongs. The metal powder collected is mixed with some zinc oxide and is known as “zinc dust”. Some of the zinc metal is collected in the fused state. This is solidified in moulds. This metal is called “zinc spelter”.
ZnO + C → Zn + CO
ZnO + CO → Zn + CO2
Electrolytic refining : Very pure zinc is obtained by electrolysis. The electrolyte is zinc sulphate solution containing a little H2SO4. Impure zinc is made the anode and the pure zinc plates are made the cathode.
Question 17.
Explain smelting process in the extraction of copper.
Answer:
The roasted ore is mixed with a little coke and sand (Silica) and smelted in a blast furnace and fused. A blast of air, necessary for the combustion of coke, is blown through the tuyeres present at the base of the furnace. The oxidation of the sulphides of copper and iron will be completed further. A slag of iron silicate is formed according to the reactions given below :
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → Fe SiO3
Ferrous Silicate (Slag)
Cu2O + FeS → Cu2S + FeO
Question 18.
Explain electrometallurgy with an example.
Answer:
Electro metallurgy : Metallurgy involving the use of electric arc furnaces, electrolysis and other electrical operations is called electrometallurgy.
In the reduction of a molten metal salt, electrolysis is used. These methods are based on electro chemical principles.
These are expained by the equation
∆G° = -n FE°
n = no. of electrons
E° = Electrode potential.
Electrolytic Reduction of Alumina : Pure Alumina (Al2O3) is a bad conductor of electricity and it has high melting point (2050°C). So it cannot be electrolysed. Alumina is electrolysed by dissolving in fused cryolite to increase the conductivity and small amount of Fluorspar is added to reduce its melting point. Thus the electrolyte is a fused mixture of Alumina, Cryolite and Fluorspar.
Electrolysis is carried out in an iron tank lined inside with graphite (carbon) which functions as cathode. A number of carbon rods (or) copper rods suspended in the electrolyte functions as Anode.
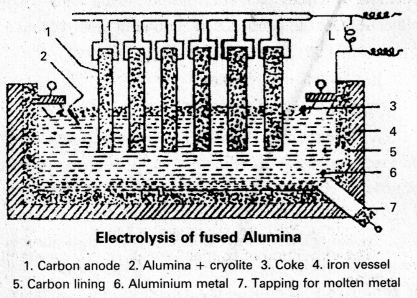
An electric current of 100 amperes at 6 to 7 volts is passed through the electrolyte. Heat produced by the current keeps the mass in fused state at 1175 to 1225K. The following reactions take place in the electrolytic cell under these conditions.
Na3AlF6 → 3NaF + AlF3
Cryolite
4AlF3 → 4Al3+ + 12F–
At cathode : 4Al3+ + 12e– → 4Al
At anode : 12F– → 6F2 + 12e–
F2 formed at the anode reacts with alumina and forms Aluminium fluoride.
2Al2O3 + 6F2 → 4AlF3 + 3O2
Aluminium, produced at the cathode, sinks to the bottom of the cell. It is removed from time to time through topping hole.
![]()
Question 19.
Explain briefly the extraction of aluminium from bauxite.
Answer:
Extraction of Aluminium from Bauxite:
For the purpose of extraction of Al, Bauxite is the best source.
purification of Bauxite : Bauxite containing Fe2O3 as impurity is known as red Bauxite.
Bauxite containing SiO2 as impurity is known as white Bauxite and can be purified by “Serpeck’s Process”. Red Bauxite is purified by Bayer’s process and Hall’s process.
Bayer’s Process : Red bauxite, is roasted and digested in concentrated NaOH at 423 K. Bauxite dissolves in NaOH to form sodium meta aluminate while impurity Fe2O3 does not dissolve which can be removed by filtration.
Al2O3.2H2O + 2NaOH → 2NaAlO2 + 3H2O
The solution which contains sodium meta aluminate is diluted and crystals of AZ(OH)3, are added which serves as seeding orgent. Sodium meta aluminate undergoes hydrolysis to precipitate Al(OH)3.
2NaAlO2 + 4H2O → 2NaOH + 2Al(OH)3 ↓
Al(OH)3 is filtered and ignited at 1200°C to get anhydrous alumina.
2Al(OH)3 ![]() Al2O3 + 3H2O
Al2O3 + 3H2O
Halls’ Process: Red Bauxite is fused with sodium carbonate to form sodium meta aluminate which is extracted with water. The impurity Fe2O3 is filtered out.
Al2O3 + Na2CO3 → 2NaAlO2 + CO2
Into the solution of sodium meta aluminate, CO2 gas is passed to precipitate Al(OH)3.
![]()
The precipitated Al(OH)3 is ignited at 1200°C to get anhydrous alumina.
2Al(OH)3 → Al2O3 + 3H2O
Serpeck’s Process: Powdered bauxite is mixed with coke and heated to 2075 K in a current of nitrogen gas. Aluminium Nitride is formed while Si02 is reduced to SiO2 which escapes out.
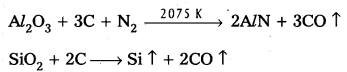
Aluminium nitride is hydrolysed to get aluminium hydroxide which on ignition gives anhydrous alumina.
AlN + 3H2O → Al(OH)3 ↓ + NH3 ↑
2Al(OH)3 ![]() 3 Al2O3 + 3H2O
3 Al2O3 + 3H2O
Electrolytic Reduction of Alumina : Pure Alumina (Al2O3) is a bad conductor of electricity and it has high melting point (2050°C). So it cannot be electrolysed. Alumina is electrolysed by dissolving in fused cryolite to increase the conductivity and small amount of Fluorspar is added to reduce its melt¬ing point. Thus the electrolyte is a fused mixture of Alumina, Cryolite and Fluorspar.
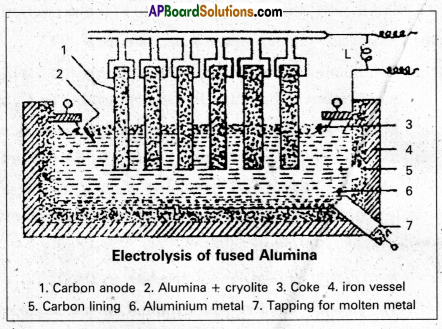
Electrolysis is carried out in an iron tank lined inside with graphite (carbon) which functions as cathode. A number of carbon rods (or) copper rods suspended in the electrolyte functions as Anode.
Na3AlF6 → 3NaF + AlF3
Cryolite
4AlF3 → 4Al3+ + 12F–
At cathode : 4Al3+ + 12e– → 4Al
At anode : 12F– → 6F2 + 12e–
F2 formed at the anode reacts with alumina and forms Aluminium fluoride.
2Al2O3 + 6F2 → 4AlF3 + 3O2
Aluminium, produced at the cathode, sinks to the bottom of the cell. It is removed from time to time through topping hole.
Purification of Aluminium : (Hoope’s Process)
The impurities present are Si, Cu, Mn etc.,
The electrolytic cell used for refining of aluminium consists of iron tank lined inside with carbon. This acts as anode. The tank contains three layers of fused masses. The bottom layer contains impure aluminium. Middle layer contains mixture of AlF3, NaF and BaF2 saturated with Al2O3. Top layer contains pure aluminium and graphite rods kept in it act as cathode.
On passing current aluminium ions from the middle layer are discharged at the cathode as pure aluminium. Equivalent amount of aluminium from the bottom layer passes into middle layer.
Long Answer Questions
Question 1.
The choice of a reducing agent in a particular case depends on thermo-dynamic factor, •lain with two examples.
Answer:
The choice of a reducing agent in a particular case depends on thermodynamic factor. This fact can be explained by considering the following examples.
- Out of C and CO, Carbon nonoxide (CO) is a better reducting agent at 673K.
- At 983K (or) above coke(C) is better reducing agent.
- The above observations are form Ellingham diagram.
- Zinc is not extracted from zinc oxide through reduction using CO.
Explanation :
2Zn + O2 → 2ZnO, ∆G° = -650 kJ
2CO + O2 → 2CO2, ∆G° = -450 kJ
2ZnO + 2CO → 2Zn + 2CO2, ∆G° = 200 kJ
∆G° = Positive indicatis that the reaction is not feasible.
The extraction of copper from pyrites is more difficult than that from its oxide ore through reduction. ‘ .
Explanation : Pyrites (Cu2S) Cannot be reduced by carbon (or) hydrogent because the standard free energy of formation (∆G°) of Cu2S is greater then those of CS2 and H2S.
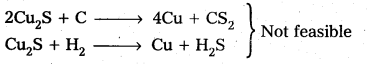
The ∆G° of copper oxide is less than that of CO2.
∴ The sulphide ore is first converted to oxide by roasting and then reduced. Roasting
The following are the conditions that Al might be expected to reduce MgO. The Equations for the formation of two oxides are
\(\frac{4}{3}\)Al(S) + O2(g) → \(\frac{2}{3}\) Al2O3(S)
2Mg(S) + O2(g) → 2MgO(S)
From the Ellingham diagram the two curves of these oxides formation intersect each other at a certain point. The corresponding value of ∆G° becomes zero for the reduction of MgO by aluminium metal,
2MgO(S) + \(\frac{4}{3}\)Al(S) → 2Mg(S) + \(\frac{2}{3}\) Al2O3(S)
- From the above information the reduction of MgO by Al metal cannot occur below this temperature (1665 K) instead, Mg can reduce Al2O3 to Al below 1665 K.
- Al-Metal can reduce MgO to Mg above 1665 K because ∆G° for Al2O3 is less compared to that of MgO.
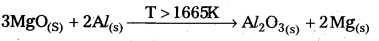
![]()
Question 2.
Discuss the extraction of Zinc from Zinc blend
Answer:
“Zinc blende (ZnS)” is an important source for ‘Zn’metal.
Extraction of’Zinc : Zinc blende ore is treated in the following states.
i) Crushing : The ore is crushed to a fine powder in ball mills.
ii) Concentration of the ore : The ore is concentrated first by gravity process. The crushed ore is washed with a stream of water on a Wilfley’s table. The tables have a corrugated top and are under a rocking motion. Due to this motion, the lighter gangue particles are washed away by the steam. The heavier ore particles settle to the bottom of the table.
The partially concentrated ore is further concentrated by froth floatation process. The ore particles go with the froth.
The concentrated ore is subjected further to electromagnetic separatioh, if iron oxide is present in the gangue. Iron oxide is magnetic and so forms a heap nearer to the magnetic pole.
iii) Roasting: The concentrated ore thus obtained is roasted in rotary shelf burner which is provided with horizontal shelves and raking arms. The ore is added at the top and zinc oxide is collected from the bottom. The following reactions take place.
2ZnS + 3O2 → 2ZnO + 2SO2
ZnS + 2O2 → ZnSO4
2ZnSO4 → 2ZnO + 2SO2 + O2
iv) Reduction : Three methods are in practice for the reduction of the oxide to the metal. The most commonly used method is the Belgian process. In this process the roasted ore is mixed with coal or coke intimately and is taken in fireclay or earthern ware retorts. The retorts are made of fireclay which are bottle shaped tubes. These are closed at one end. The other end is connected to air cooled earthern ware condenser. A large number of retorts are placed in tiers in a large furnace and heated in 1I00°C by burning the gas. ‘Prolongs’ made of sheet iron are attached to the condensers. The metal condenses in these earthern ware condensers and the prolongs. The metal powder collected is mixed with some zinc oxide and is known as “zinc dust”. Some of the zinc metal is collected in the fused state. This is solidified in moulds. This metal is called “zinc spelter”.
ZnO + C → Zn + CO
ZnO + CO → Zn + CO2
Electrolytic refining : Very pure zinc is obtained by electrolysis. The electrolyte is zinc sulphate solution containing a little H2SO4. Impure zinc is made the anode and the pure zinc plates are made the cathode.
Question 3.
Explain the reactions occuring in the blast furnace in the extraction of iron.
Answer:
In the Blast furnace, reduction of iron oxides takes place in different temperature ranges. Hot air is blown from the bottom of the furnace and coke is burnt to give temperature upto about 2200K in the lower portion itself. The burning of coke therefore supplies most of the heat required in the process. The CO and heat moves to upper part of the furnace. In upper part, the temperature is 1o%er and the iron oxides (Fe2O3 and Fe3O4) coming from the top are reduced in steps to FeO. Thus, he reduction reactions taking place in the lower temperature range and in the higher temperature range, depend on the points of corresponding intersections in the ∆rG° vs T plots.
These reactions can be summarised as follows:
At 500 – 800 K (lower temperature range in the blast furnace)
3 Fe2O3 + CO → 2 Fe3O4 + CO2
Fe3O4 + 4 CO → 3 Fe + 4 CO2
Fe2O3 + CO → 2 FeO + CO2
At 900 – 1500 K (higher temperature range in the blast furnace)
C + CO2 → 2 CO
FeO + CO → Fe + CO2
Lime stone is also decomposed to CaO which removes silicate impurity of the ore as CaSiO3 slag. The slag is in molten state and separates out from iron.
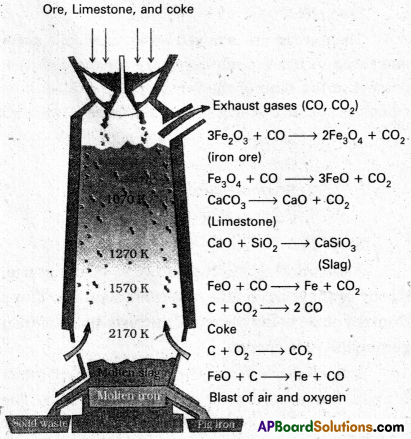
The iron obtained from Blast furnace contains about 4% carbon and many impurities in smaller amount (e.g., S, P, Si, Mn). This is known as pig iron. Cast iron is different from and is made by melting pig iron with scrap iron and coke using hot air blast. It has slightly lower carbon content (about 3%) and is extremely hard and brittle.
Fe2O3 3 C → 2 Fe + 3 CO
![]()
Question 4.
Discuss the extraction of copper from copper pyrites.
Answer:
Extraction of copper from copper pyrites:
Copper pyrite is the main source of copper metal. Various steps involved in the extraction of copper discussed below.
Step – I:
Concentration of ore by froth floatation process:
The ore is first crushed in ball mills. The finely divided ore is suspended in water. A little pine oil is added and the mixture is vigorously agitated by a current of air. The froth formed carries the ore particles almost completely. The gangue sinks to the bottom of the tank. The froth is separated and about 95% concentrated ore is obtained.
Step – II: Roasting :
To remove the volatile impurities like As (or) Sb, the ore is roasted in a free supply of air. A mixture of sulphides of copper and iron are obtained and these are partially oxidised to respective oxides.
Cu2S. Fe2S3 + O2 → Cu2S + 2FeS + SO2
2Cu2S + 3O2 → 2Cu2O + 2SO2
2FeS + 3O2 → 2FeO + 2SO2
Step – III :
The roasted ore is mixed with a little coke and sand (Silica) and smelted in a blast furnace and fused. A blast of air, necessary for the combustion of coke, is blown through the tuyeres present at the base of the furnace. The oxidation of the sulphides of copper and iron will be completed further. A slag of iron silicate is formed according to the reactions given below :
2FeS + 3O2 → 2FeO + 2SO2
FeO + SiO2 → Fe SiO3
Ferrous Silicate (Slag)
Cu2O + FeS → Cu2S + FeO
Step -IV:
After smelting the copper ore in blast furnace, the product of the blast furnace consists mostly of Cu2S and a little of ferrous sulphide. This product is known as “Matte.” It is collected from the outlet at the bottom of the furnace . After then the following processes are carried out for getting the pure copper.
1. Bessemerization: The matte is charged into a Bessemer converter. A bessemer converter is a pear-shaped furnace. It is made of steel plates. The furnace is given a basic lining with lime or magnesium oxide (obtained from dolomite or magnesite). The converter is held in position by trunnions and can be tilted in any position. A hot blast of air and sand is blown through the tuyeres present near the bottom. Molten metal, the product in the furnace, collects at the bottom of the converter.
Reactions that took place in blast furnace go to completion. Almost all of iron is eliminated as a slag. Cuprous oxide combines with cuprous sulphide and forms Cu metal.
2Cu2O + Cu2S → 6Cu + SO2
The molten metal is cooled in sand moulds. S02 escapes. The impure copper metal is known as “Blister copper” and is about 98% pure.
Step -V:
Refining : The Blister copper is purified by electrolysis. The impure copper metal is made into plates. They are suspended into lead – lined tanks containing Copper (II) Sulphate solution. Thin plates of pure copper serve as cathode. The cathode plates are coated with graphite. On electrolysis, pure copper is deposited at the cathode. The copper obtained is almost 100% pure Cu.
![]()
Question 5.
Explain the various steps involved in the extraction of almninium from bauxite.
Answer:
Extraction of Aluminium from Bauxite:
For the purpose of extraction of Al, Bauxite is the best source.
purification of Bauxite : Bauxite containing Fe2O3 as impurity is known as red Bauxite.
Bauxite containing SiO2 as impurity is known as white Bauxite and can be purified by “Serpeck’s Process”. Red Bauxite is purified by Bayer’s process and Hall’s process.
Bayer’s Process : Red bauxite, is roasted and digested in concentrated NaOH at 423 K. Bauxite dissolves in NaOH to form sodium meta aluminate while impurity Fe2O3 does not dissolve which can be removed by filtration.
Al2O3.2H2O + 2NaOH → 2NaAlO2 + 3H2O
The solution which contains sodium meta aluminate is diluted and crystals of AZ(OH)3, are added which serves as seeding orgent. Sodium meta aluminate undergoes hydrolysis to precipitate Al(OH)3.
2NaAlO2 + 4H2O → 2NaOH + 2Al(OH)3 ↓
Al(OH)3 is filtered and ignited at 1200°C to get anhydrous alumina.
2Al(OH)3 ![]() Al2O3 + 3H2O
Al2O3 + 3H2O
Halls’ Process: Red Bauxite is fused with sodium carbonate to form sodium meta aluminate which is extracted with water. The impurity Fe2O3 is filtered out.
Al2O3 + Na2CO3 → 2NaAlO2 + CO2
Into the solution of sodium meta aluminate, CO2 gas is passed to precipitate Al(OH)3.
![]()
The precipitated Al(OH)3 is ignited at 1200°C to get anhydrous alumina.
2Al(OH)3 → Al2O3 + 3H2O
Serpeck’s Process: Powdered bauxite is mixed with coke and heated to 2075 K in a current of nitrogen gas. Aluminium Nitride is formed while Si02 is reduced to SiO2 which escapes out.
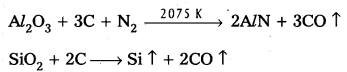
Aluminium nitride is hydrolysed to get aluminium hydroxide which on ignition gives anhydrous alumina.
AlN + 3H2O → Al(OH)3 ↓ + NH3 ↑
2Al(OH)3 ![]() 3 Al2O3 + 3H2O
3 Al2O3 + 3H2O
Electrolytic Reduction of Alumina : Pure Alumina (Al2O3) is a bad conductor of electricity and it has high melting point (2050°C). So it cannot be electrolysed. Alumina is electrolysed by dissolving in fused cryolite to increase the conductivity and small amount of Fluorspar is added to reduce its melt¬ing point. Thus the electrolyte is a fused mixture of Alumina, Cryolite and Fluorspar.

Electrolysis is carried out in an iron tank lined inside with graphite (carbon) which functions as cathode. A number of carbon rods (or) copper rods suspended in the electrolyte functions as Anode.
Na3AlF6 → 3NaF + AlF3
Cryolite
4AlF3 → 4Al3+ + 12F–
At cathode : 4Al3+ + 12e– → 4Al
At anode : 12F– → 6F2 + 12e–
F2 formed at the anode reacts with alumina and forms Aluminium fluoride.
2Al2O3 + 6F2 → 4AlF3 + 3O2
Aluminium, produced at the cathode, sinks to the bottom of the cell. It is removed from time to time through topping hole.
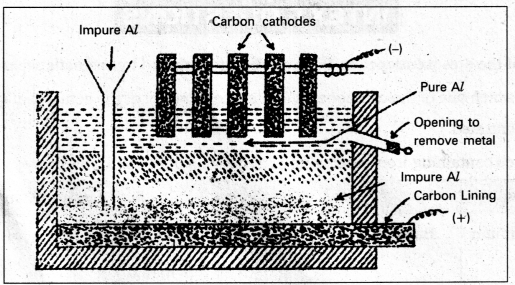
Purification of Aluminium : (Hoope’s Process)
The impurities present are Si, Cu, Mn etc.,
The electrolytic cell used for refining of aluminium consists of iron tank lined inside with carbon. This acts as anode. The tank contains three layers of fused masses. The bottom layer contains impure aluminium. Middle layer contains mixture of AlF3, NaF and BaF2 saturated with Al2O3. Top layer contains pure aluminium and graphite rods kept in it act as cathode.

On passing current aluminium ions from the middle layer are discharged at the cathode as pure aluminium. Equivalent amount of aluminium from the bottom layer passes into middle layer.
Textual Examples
Question 1.
Suggest a condition under which magnesium could reduce alumina.
Answer:
The two equations are :
a) \(\frac{4}{3}\) Al + O2 → \(\frac{2}{3}\) Al2O3
b) 2 Mg + O2 → 2MgO
At the point of intersection of the Al2O3 and MgO curves (marked. “A” in Ellingham diagram), the ∆G⊖ becomes ZERO for the reaction :
\(\frac{2}{3}\) Al2O3 + 2Mg → 2MgO + \(\frac{4}{3}\) Al
Below that point magnesium can reduce alumina.
Question 2.
Although thermodynamically feasible, in practice, magnesium metal is not used for the reduction of alumina in the metallurgy of aluminium. Why ?
Answer:
Temperatures below the point of intersection of Al2O3 and MgO curves, magnesium can reduce alumina. But the process will be uneconomical.
![]()
Question 3.
Why is the reduction of a metal oxide easier if the metal formed is in liquid state at the temperature of reduction ?
Solution:
The entropy is higher if the metal is in liquid state than when it is in solid state. The value of entropy change (∆S) of the reduction process is more on +ve side when the metal formed is in liquid state and the metal oxide being reduced is in solid state. Thus the value of ∆G⊖becomes more on negative side and the reduction becomes easier.
Question 4.
At a site, low grade copper ores are available and zinc and iron scraps are also available. Which of the two scraps would be more suitable for reducing the leached copper ore and why ?
Solution:
Zinc being above iron in the electrochemical series (more reactive metal is zinc), the reduction will be faster in case zinc scraps are used. But zinc is costlier metal than iron. So using iron scraps will be advisable and advantageous.
Intext Questions
Question 1.
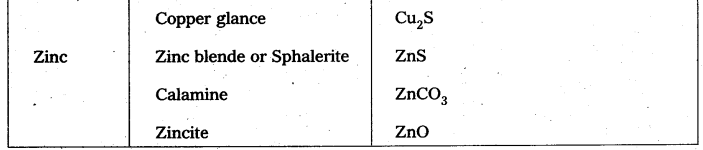
Which of the ores mentioned in Table can be concentrated by magnetic separation method ?
Answer:
Ores in which one of the components (either the impurity or the actual ore) is magnetic can be concentrated.
E.g.: ores containing iron (haematite, magnetite, siderite and iron pyrites).
Question 2.
What is the significance of leaching in the extraction of aluminium ?
Answer:
Leaching is significant as it helps in removing the impurities like SiO2, Fe2O3 etc. from the bauxite ore.
Question 3.
The reaction, Cr2O3 + 2 Al → Al2O3 + 2 Cr (∆G⊖ = -421 kJ) is thermodynamically feasible as is apparent from the Gibbs energy value. Why does it not take place at room temperature?
Answer:
Certain amount of activation energy is essential even for such reactions which are thermodynamically feasible, therefore heating is required.
![]()
Question 4.
Is it true that under certain conditions Mg can reduce Al2O3 and Al can reduce MgO ? What are those conditions ?
Answer:
Yes, below 1350°C Mg can reduce Al2O3 and above 1350°C, Al can reduce MgO. This can be inferred from ∆G⊖ vs T plots.