Students can go through AP State Board 10th Class Biology Notes Chapter 7 Coordination in Life Processes to understand and remember the concept easily.
AP State Board Syllabus 10th Class Biology Notes Chapter 7 Coordination in Life Processes
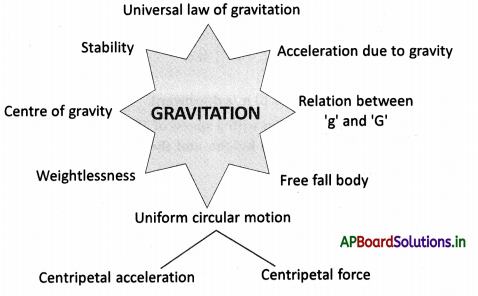
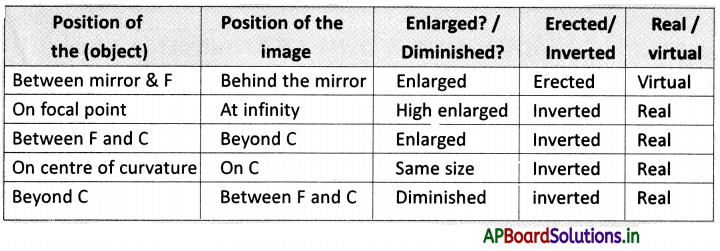
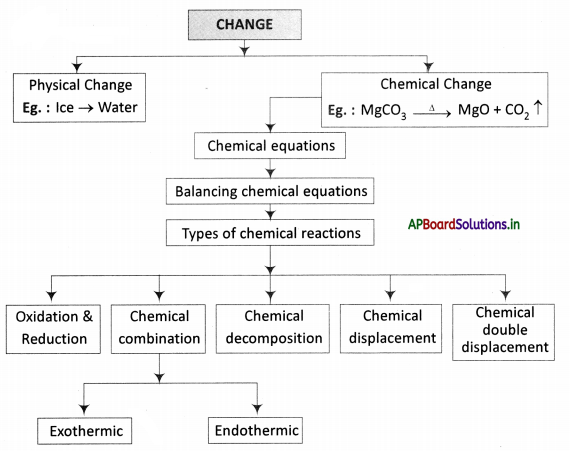
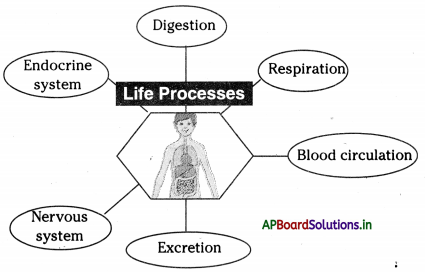
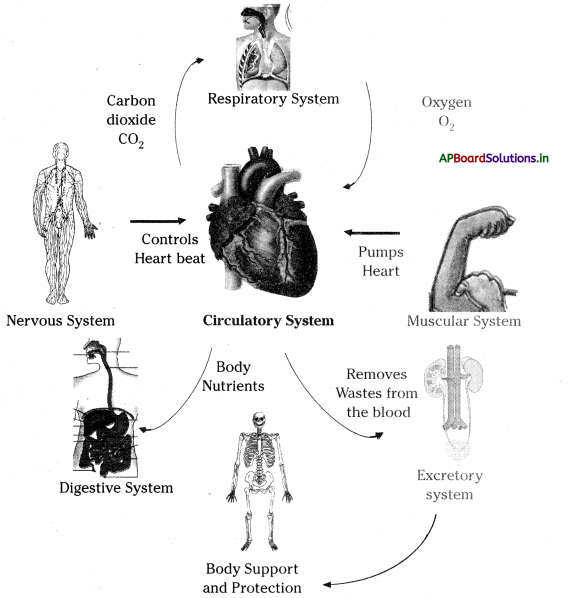
→ Every life process is dependent on others to keep the body in good condition.
→ When glucose levels in the blood fall we get hunger pangs in the stomach.
→ The hormone ghrelin secreted in the stomach is responsible for hunger generating sensations.
→ Ghrelin is secreted from certain cells in the wall of the stomach.
→ Hormone leptin suppresses hunger.
→ Interactions between the senses of taste and smell enhance our perceptions of the foods we eat.
→ Russian scientist Ivan Pavlov has conducted experiments on conditioned reflexes.
→ Taste can be identified easily when the tongue is pressed against the palate.
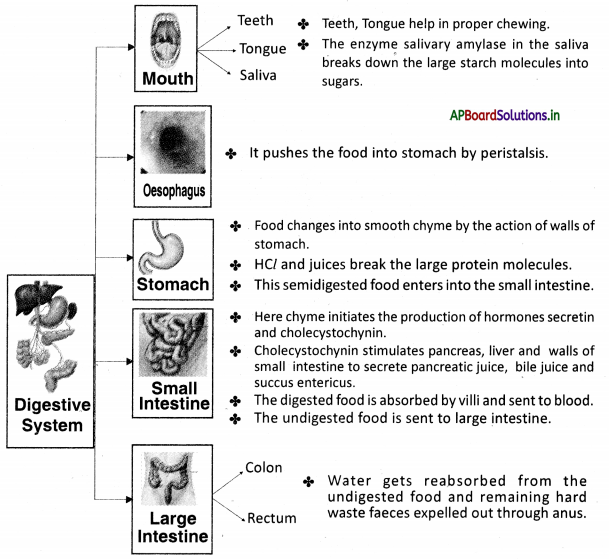
→ Food in the mouth has to be broken down into tiny pieces to increase the surface area for the action of substances that aid in digestion.
→ The dental formula of human beings is \(\frac{2,1,2,3}{2,1,2,3}\)
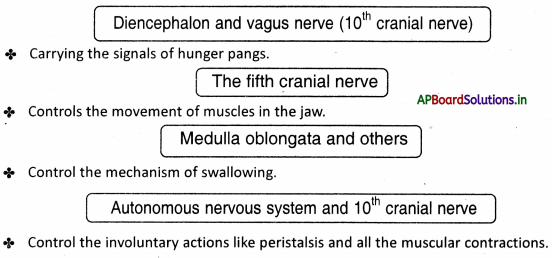
→ The fifth cranial nerve controls the movement of muscles in the jaw.
→ The mechanism for swallowing is also under nervous coordination and its control center is in the brain stem.
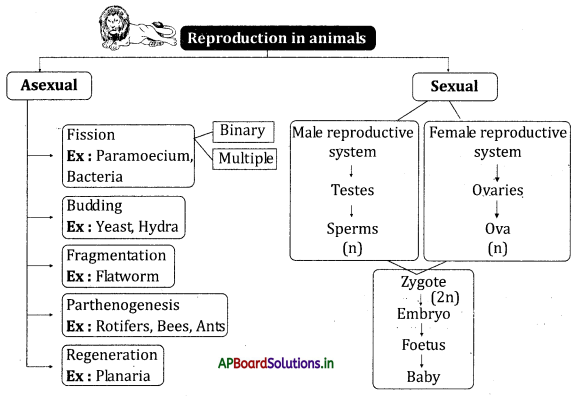
![]()
→ The nature of saliva is alkaline.
→ Saliva is secreted by the action of the autonomous nervous system from salivary glands. The chewing of food in the mouth forms a slurry mass called a bolus.
→ The wall of the esophagus is made up of inner layer circular muscles and the outer layer longitudinal muscles.
→ Contraction and relaxation of the muscles present in the esophagus resulting in wave-like movements called peristaltic movements.
→ The food from the esophagus reaches the stomach by peristaltic movements.
→ The partially digested food present in the stomach is known as chyme.
→ Pyloric sphincter present at the opening of the stomach and small intestine releases a small amount of chyme into the duodenum.
→ Reverse peristalsis occurs in ruminating animals like cows.
→ The gastric juice produced by the stomach contains mucus, hydrochloric acid, and pepsin. Food is completely digested in the small intestine.
→ Absorption of nutrients by villi takes place in the small intestine.
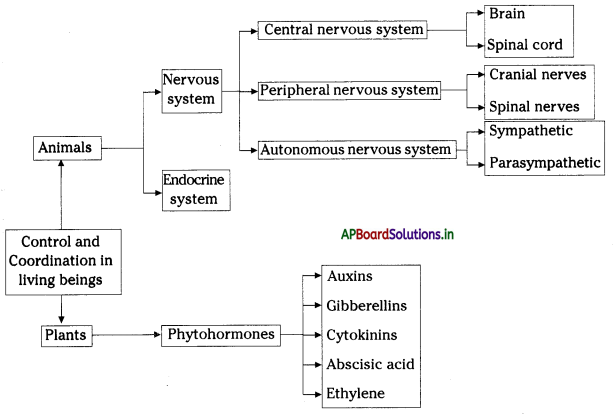
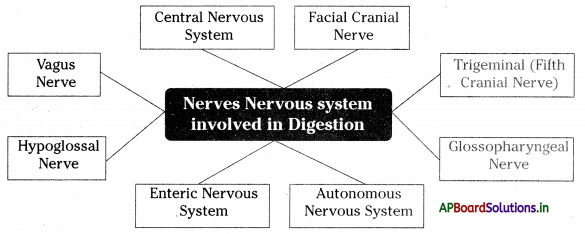
→ The neural apparatus of our digestive tract comprises a vast and complicated network of neurons called the second brain or enteric nervous system.
→ The enteric nervous system stimulates and coordinates the breakdown of food, absorbing nutrients and expelling wastes from the body.
→ We secrete around 1 to 1.5 liters of saliva per day.
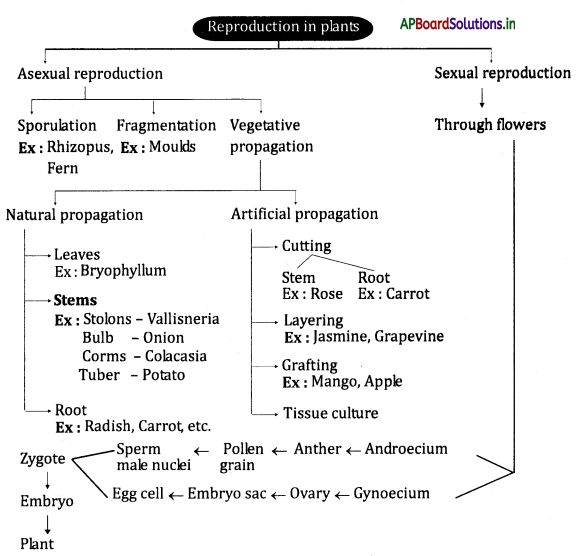
→ Oxidation of food is required to release energy.
![]()
→ Respiration is controlled by the medulla oblongata of the autonomous nervous system.
→ Digestion occurs in the food canal, coordination of respiration and blood circulation is necessary otherwise oxidation of food and transport of substances will not take place.
→ Ghrelin: It is a hormone produced mainly by the cells lining the human stomach and cells of the pancreas which stimulate hunger.
→ Leptin: It is the hormone that suppresses hunger.
→ Gustatory: Concerned with tasting or the sense of taste.
→ Chemoreceptors: Sensory cells or organs responsive to chemical stimuli.
→ Papillae: One of the certain small protuberances concerned with the senses of touch, taste, and smell.
→ Food Bolus: A small rounded mass of substance, especially of chewed food at the moment of swallowing.
→ Peristalsis: Contraction and relaxation of circular and longitudinal muscles in the esophagus bring a wave Like motion that propels the food bolus into the stomach by the action called peristalsis.
→ Chyme: The partly digested food in the stomach.
→ Pyloric Sphincter: Muscles present at the opening of the stomach and the first part of the small intestine and duodenum.
→ Villi: These are tiny finger-like projections that enable the small intestine to absorb nutrients from food.
→ Medulla oblongata: The continuation of the spinal cord within the skull, forming the lowest part of the brain stem and containing control centers for the heart and lungs.
→ Brain stem: The central trunk of the mammalian brain, consisting of the medulla oblongata, Ponsvaroll, and midbrain.
→ Hunger pangs: These are the contractions made by the stomach when we feel hungry.
→ Stale food: Food that is not freshly prepared but not harmful. It may not taste good.
![]()
→ Sipping: To drink a liquid by taking small mouthfuls.
→ Relish: Liking or enjoyment of the taste of something.
→ Palate: The roof of the mouth, separating the cavities of the mouth and nose invertebrates.
→ Munch: To chew with the steady or vigorous working of the jaws, often audibly.
→ MasticatIon: Biting and grinding food in our mouth so it becomes soft enough to swallow.
→ Belching: It is the emission of wind noisily from the stomach through the mouth.
→ PorrIdge: It is a dish made by boiling ground, crushed or chopped cereal In water, milk, or both.
→ Rumination: The process of rechewing the cud to further break down plant matter and stimulate digestion is called rumination.
→ Succus entericus: Intestinal juice secreted by glands lining the small intestinal walls. It continues downwards to form the spinal cord.
→ Neurotransmitter: A chemical substance that is released at the end of a nerve fiber by the arrival of a nerve impulse and by diffusing across the synapse and junction. It affects the transfer of the impulse to another nerve fiber, muscle fiber, or some other structure.
![]()
→ Second brain (or) Enteric nervous system: One of the main divisions of the autonomic nervous system and consists of a mesh-like system of neurons that governs the function of the gastrointestinal system.
→ Colon: It is the last part of the digestive system in most vertebrates. It removes water, salt, and some nutrients forming stool.
→ Anal sphincter: Either of two sphincters controlling the closing of the anus.
![]()
→ Ivan Petrovich Pavlov [1849 – 1936]:
- Ivan Petrovich Pavlov [1849 – 1936] was a famous Russian physiologist.
- He devoted his life to the study of physiology and sciences, making several remarkable discoveries and ideas that were passed on from generation to generation.
- Inspired when the progressive ideas which D.l. Pisarev, the most eminent of the Russian literary critics of the 1860s and I. M. Sechenov, the father of Russian physiology, were spreading, Pavlov abandoned his religious career and decided to devote his life to science.
- In 1870 he enrolled In the physics and mathematics faculty at the University of Saint Petersburg to take the course in natural science.
- He won the Nobel Prize for Physiology or Medicine in 1904.