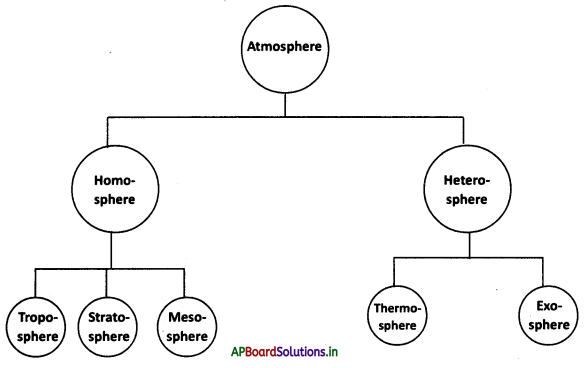
Students can go through AP State Board 10th Class Physical Science Notes Chapter 8 Chemical Bonding to understand and remember the concept easily.
AP State Board Syllabus 10th Class Physical Science Notes Chapter 8 Chemical Bonding
→ A chemical bond is an attractive force between two atoms in a molecule.
→ Atoms which contain 8 electrons in the outermost shell are stable and less reactive.
→ Electron transfer occurs from one atom to another because atoms try to acquire an inert gas configuration.
→ Ions are charged particles.
→ The atom which loses electron acquire positive charge and the atoms gain electrons acquire a negative charge.
→ The formation of ionic compounds involves the formation of ions and the attraction of ion pairs.
→ Oxidation involves the removal of electrons.
→ Reduction involves the addition of electrons.
→ Oxidation and reduction go side by side.
→ The compounds containing molecules that are formed by sharing of electrons are known as covalent compounds.

→ Covalent compounds are soft with low melting and boiling points.
→ Molecules having two oppositely charged poles are called polar molecules.
→ Vander Waal’s forces are purely electrostatic forces operating between molecules at a short distance.
→ A dipolar molecule attracting announcer dipolar molecule is called dipole-dipole attraction.
→ A strong bond is formed by the maximum overlap of orbitals.
→ End-end overlap of orbitals leads to the formation of sigma (σ) bond and part overlap of orbitals leads to the formation of pi (π) bond.
→ a bond is stronger than an π bond.
→ σ (Sigma) bond exists independently.
→ π bond has no independent existence. It exists only in the presence of a sigma bond.
→ In a double bond one, a and one π bond are present.

→ In a triple bond, one d and two π bonds are present.
→ s – s overlap is present in H2.
→ p – p overlap is present in F2, Cl2, Br2, and I2.
→ s – p overlap is present in HF, HC/, HBr, and HI.
→ Molecules having single bonds have only o bonds.
→ Molecules having a single bond are H2, F2, Cl2, Br2, I2, BeCl2, etc.
→ Molecules having double bonds are O2, C2H4, CO2, etc.
→ C2H2, IM2, HCN, CaC2, etc. have triple bonds.
→ The number of valence electrons available in the atoms decides the type of bond.
→ To explain the bond angles in the molecules through covalent bonds the Valence – Shell – Electron – Pair – Repulsion – Theory (VSEPR).
→ VSEPR was proposed by Sidgwickand Powell (1940). It was further improved by Gillespie and Nyholm (1957).
→ Electrons: Elementary particles in an atom with a negative charge.
→ Noble gases: A group of monoatomic gaseous elements forming group ‘O’ of the periodic table.
→ Lewis dot structure: The valence electrons in the atom of an element are depicted in a short form by Lewis symbol or Lewis dot structure.
→ Octet rule: A stable group of eight electrons in the outer shell of an atom is called the octet rule.
→ Chemical bond: The force of attraction between any two atoms or a group of atoms that results in a stable entity is called a chemical bond.
→ Ionic bond: The electrostatic attraction force that keeps cation and anion together to form a new electrically neutral compound is called ‘Ionic bond’.

→ Covalent bond: The chemical bond formed between two atoms by mutual sharing of a pair of valence shell electrons so that both of them can attain octet or duplet in their valence shell is called the covalent bond.
→ Cation: It is a positively charged ion formed by the removal of an electron from an atom.
→ Anion: It is a negatively charged ion formed by the addition of an electron to the atom.
→ Electrostatic force: Force of attraction between positively charged and negatively charged bodies or ions is called electrostatic force.
→ Electrovalent: The valence concept has been explained in terms of electrons. It is also called electrovalent.
→ Polar solvent: Polar solvents are the compounds such as water and liquid ammonia which have dipole moments and consequently high dielectric constants. The solvents are capable of dissolving ionic compounds or covalent compounds that ionize.
→ Non-polar solvent: Non-polar solvents are compounds such ethane and benzene, which do not have dipole moments. These do not dissolve in but will dissolve nonpolar covalent compounds
→ Formation of molecules: Atoms combine and form molecules. This is called the formation of molecules.
→ Ionic compounds: The compounds formed by the ionic bonds are called compounds.
→ Covalent compounds: The compounds formed by the covalent bonds are covalent compounds.
→ Electropositive character: The ability to lose electrons and become a positively charged ion of an atom is called the electropositive character.
→ Electronegative character: The tendency of an atom to gain electrons and become a negative ion.
→ Polar bonds: These are formed due to unequal sharing of electrons by the combining atoms.
→ Bonded pair of electrons: Electrons which arranged by atoms to share and form a bond is called bonded pair of electrons.
→ Lone pair: Unshared electron pair or non-bonding electron pair.

→ Bond length: Bond length or bond distance is the equilibrium distance between the nuclei of two atoms that form a covalent bond.
→ Bond energy: An amount of energy associated with a bond in a chemical compound.
→ The shape of the molecules: While atoms combine and form molecules they should have some shape. They are called the shape of the molecules.
→ Linear: A molecule in which the atoms are in a straight line as in carbon dioxide O = C = O.
→ Tetrahedral: Having four faces.
→ Properties of Ionic and: Formula mass, physical appearance, type of bond, covalent compounds melting point, boiling point, solubility, etc.
→ Configuration: The arrangement of electrons around the nudes in an atom.
→ Oxidation: The process in which an atom, ion, or molecule loses electrons.
→ Conductivity: Covalent compounds are poor conductors of electricity due to rigid bonding.
→ Van der Waal forces: Van der Waal forces are purely electrostatic forces operating between molecules at a short distance.
→ Dipole – dIpole forces: A dipolar molecule attracting another dipolar molecule.
→ Banana bond: Informal name for the type of electron-deficient bond holding the B – H – B bridges in boranes and similar compounds.
→ Bivalent or divalent: Having a valency of two.
→ Bisulfate: Hydrogen sulfate.
→ Alkali: A base that dissolves in water to give hydroxide ions.
→ Atom: The smallest part of an element that can exist chemically.

→ Single bond: Covalent bonds in which one pair of electrons is shared are known as covalent ‘single bond’.
→ Double bond: Covalent bonds in which two pairs of electrons are shared are known as covalent ‘double bonds’.
→ Triple bond: Covalent bonds in which three pairs of electrons are shared are known as covalent ‘triple bonds’.
→ Compound: A substance formed by the combination of elements in fixed proportions.
→ Dissociation: The breakdown of a molecule, ion, etc. into smaller molecules, ions, etc.
→ Electronegativity: It is the ability of the bonded atom to attract the electron density of the shared electrons.

→ Count Alessandro Volta (1745 – 1827):
- Volta was born in Como, Italy to a noble family.
- He made discoveries In electrostatic, meteorology, and pneumatics.
- His most famous invention, however, is the first battery (cell).
- Volta’s battery was later refined by other scientists and the French emperor, Nepolean, made volta a TMCount” for his discovery.
![]()
![]()