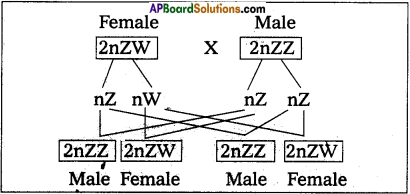
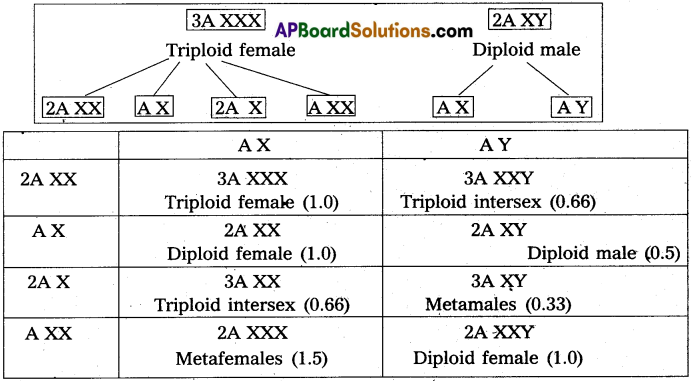
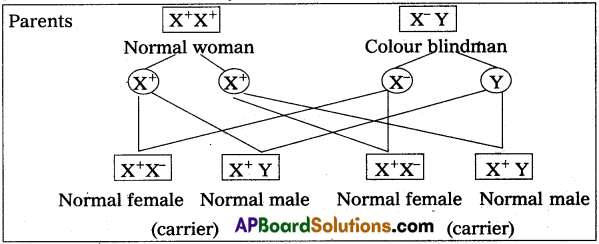
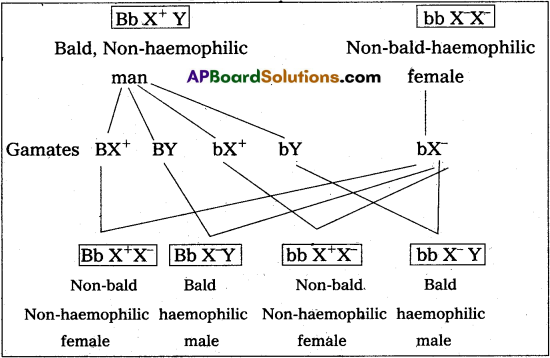
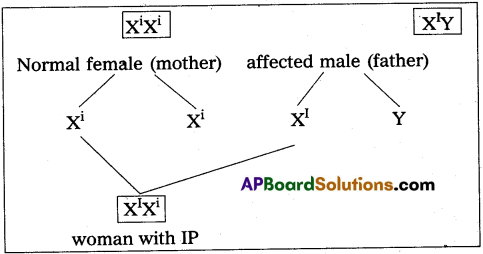
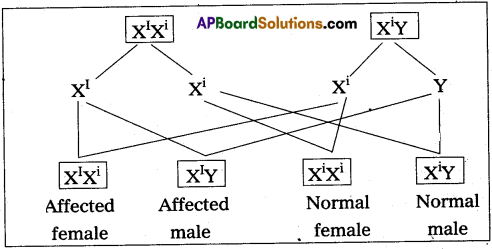
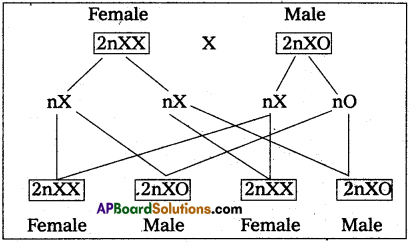
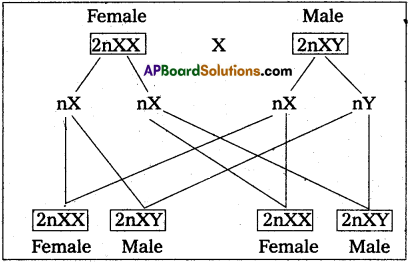
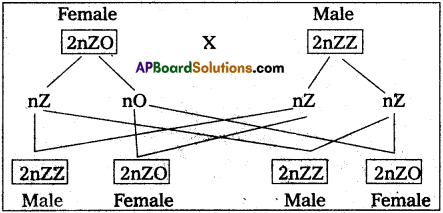
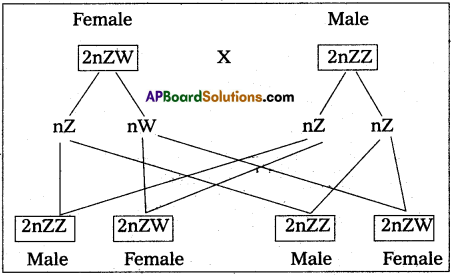
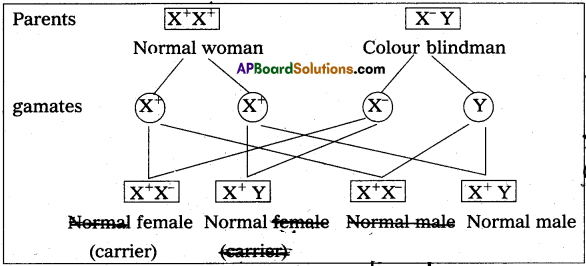
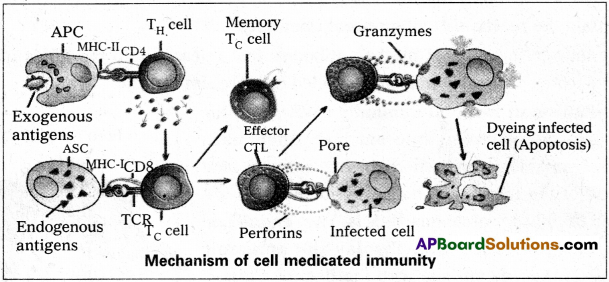
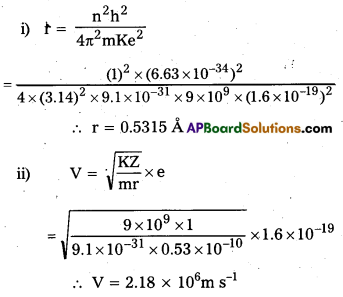
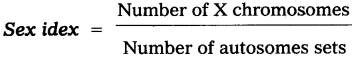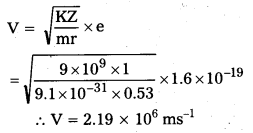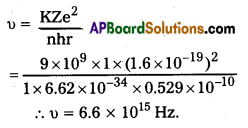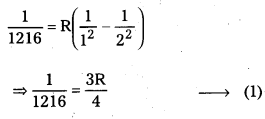Andhra Pradesh BIEAP AP Inter 2nd Year Zoology Study Material 8th Lesson Applied Biology Textbook Questions and Answers.
AP Inter 2nd Year Zoology Study Material 8th Lesson Applied Biology
Very Short Answer Questions
Question 1.
What are the factors constitute dairying?
Answer:
- Selection of good breeds having high yielding potential, combined with disease resistance ones.
- Proper housing with adequate water, feed, ventilation suitable temperature, etc.
Question 2.
Mention any two advantages of inbreeding.
Answer:
- Inbreeding increases homozygosity. Thus inbreeding is necessary if we want to evolve a pure line animal.
- It helps in the accumulation of superior genes and the elimination of less desirable genes.
Question 3.
Distinguish between out-cross and cross-breed.
Answer:
Out cross :
The offspring formed by mating of animals within the same breed, but having no ancestors on either side of pedigree for 4-6 generations.
A single out cross helps to overcome inbreeding depression.
Cross breed :
The offspring formed by a mating between superior males of one breed and superior females another breed.
Cross breed shows desirable qualities of two different breeds to be combined.
Question 4.
Define the terms layer and broiler.
Answer:
Layer :
The birds which are raised exclusively for the production of eggs are called layers.
Boiler :
The birds which are raised only for their meat are called broilers.
Question 5.
What is apiculture?
Answer:
Apiculture is the maintenance of hives of honeybees for the production of honey and wax.
Apiculture is an age-old cottage industry.
![]()
Question 6.
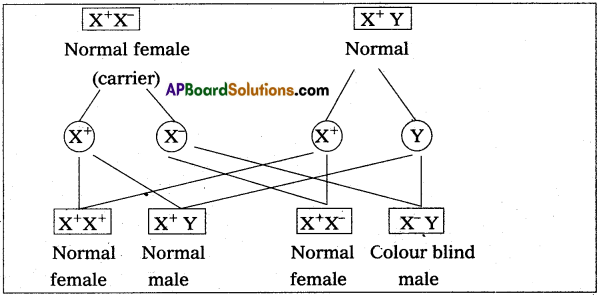
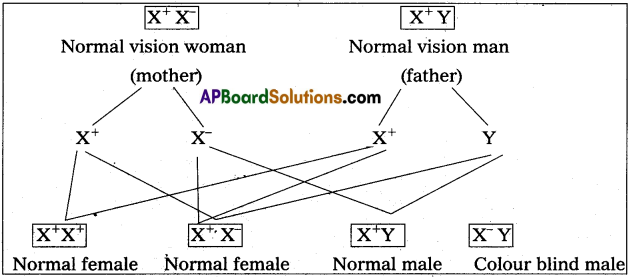
Distinguish between a drone and worker in honey bee colony.
Answer:
| Drones | worker bees |
| 1) These are fertile males. | 1) These are sterile female. |
| 2) These are developed from unfertilized ova by male parthenogenesis. | 2) These are developed from fertilized eggs. |
| 3) These are short lived. | 3) These live for two and three months. |
Question 7.
Define the term Fishery.
Answer:
Fishery is an industry devoted to the catching, processing for storage in freezers and selling of fish, shellfish or any other aquatic animals for human consumption.
Question 8.
Differentiate aquaculture and pisciculture.
Answer:
| Aquaculture | Pisciculture |
| Culturing of fishes and other aquatic organisms under regulated conditions to achieve better production. | Culturing of exclusively fin fishes under regulated conditions to achieve better production. |
Question 9.
Explain the term hypophysation.
Answer:
Making the fishds to breed artificially to meet the demand of carpseed as called hypophysation.
Question 10.
List out any two Indian carps and two exotic carps.
Answer:
Indian carps :
- Catla catla (catla)
- Cirrhinus mrigala (mrigal)
Exotic carps :
- Grass carp
- Silver carp
Question 11.
Mention any four fish by-products.
Answer:
- Shark and cod liver oils
- Fish guano
- Shagreen
- Isinglass.
Question 12.
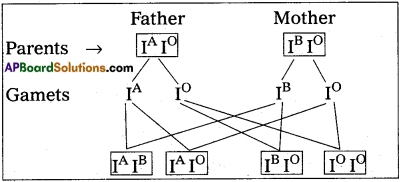
How many aminoacids and polypeptide chains are present in insulin?
Answer:
Human insulin is made up of 51 aminoacids arranged in two polypeptides.
– polypeptide chain A with 21 aminoacids
– Polypeptide chain B with 30 aminoacids.
Which are held together by disulphide linkages.
Question 13.
Define the term vaccine.
Answer:
Vaccine is biological preparation that improves immunity to a particular disease. A vaccine typically contains live attenuated an inactivated disease causing organism. The toxins or one of the surface proteins of pathogens are also used in the preparation of vaccines.
Question 14.
Mention any two features of PCR.
Answer:
- Very low concentration of bacteria or viruses can be detected by amplification of their nucleic acids by PCR.
- PCR helps to detect very low amounts of DNA by amplification of the small DNA fragments.
PCR is now routinely used for detection of HIV in suspected cases, detection of mutations and genetic disorders.
Question 15.
What does ADA strand for? Deficiency of ADA causes which disease?
Answer:
ADA stands for adenosine deaminase. Deficiency of adenosine deaminase (ADA) causes severe combined immuno deficiency (SCID).
Question 16.
Define the term transgenic animal.
Answer:
Animals that have their own genome and had their DNA manipulated to possess and express an extra or foreign gene is known as transgenic animals.
Question 17.
What is popularly called “Guardian anger of Cell Genome?
Answer:
The protein p53 is a tumor suppressor protein, which plays an important role with reference to the ”G1 check point”. In the regulation of cell division cycle. It guards the integrity of the DNA. So it is also called guardian angel of cell’s genome.
Question 18.
List out any four features of cancer cells.
Answer:
- Loss of contact inhibition
- Reduced intra cellular adhesion
- Immortalization
- Loss of anchorage dependence
Question 19.
How do we obtain radiographs?
Answer:
A beam of X-rays is produced by an X-ray generator and is projected on the body parts. X-rays that pass through the body parts are recorded on a photographic film. Photographs developed using X-rays are known as radiographs.
![]()
Question 20.
What is tomogram?
Answer:
Tomogram is a recorded image formed by computed tomography which shows the 3-D cross sectional pictures of the part of the body and displays the picture on the screen.
Question 21.
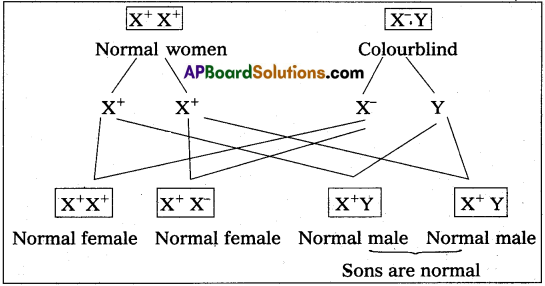
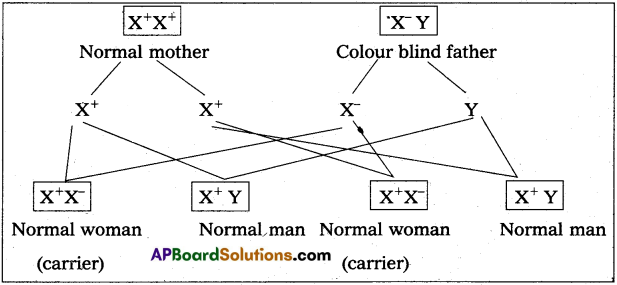
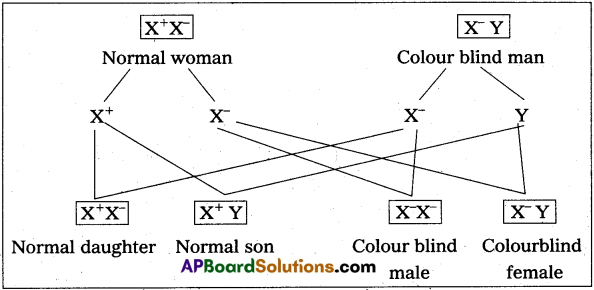
MRI scan is harmless. Justify.
Answer:
MRI does not use ionizing radiation, as involved in X-rays, and is generally safe and harmless procedure.
Question 22.
What is electrocardiography and what are the normal components of ECG?
Answer:
Electrocardiography is a commonly used, non invasive procedure for recording electrical changes in the heart.
Normal components of ECG:
(i) Waves (ii) Intervals (iii) Segments (iv) Complexes.
Question 23.
What does prolonged F-R interval indicate?
Answer:
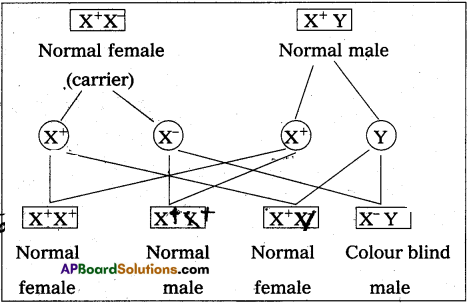
Prolonged P-R interval indicates delay in conduction of impulses from S-A node to the A-V node.
P-R interval is prolonged in bradycardia.
Question 24.
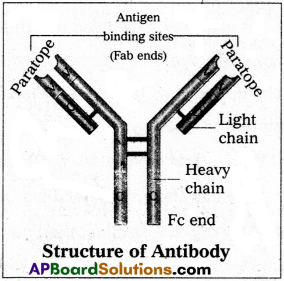
Differentiate between primary and secondary antibodies.
Answer:
| Primary antibodies | Secondary antibodies |
| 1) These antibodies are formed against the specific antigen. | 1) These antibodies are formed against the foreign primary antibody. |
| 2) These antibodies reacts with the antigens of interest. | 2) These antibodies react with the primary antibodies. |
Question 25.
Which substances in a sample are detected by direct and indirect ELISA respectively.
Answer:
- Direct ELISA – used to detect antigens present in the sample.
- Indirect ELISA – used to detect antibodies present in the sample.
Short Answer Questions
Question 1.
What are the various methods employed in animal breeding to improve livestock?
Answer:
Animal breeding is the method of mating closely related individuals.
There are broadly two methods in animal breeding. (1) In breeding (2) Out breeding
1) In breeding:
When crossing is done between animals of the same breed it is called in breeding. In breeding is of two types (a) Close breeding (b) Line breeding.
a) Close breeding:
Close breeding is mating between male parent and female offspring and/or female with male offspring.
b) Line breeding :
Line breeding is the selective breeding of animals for a desired feature by mating them within a closely related line. It leads to upgrading of a desired commercial character.
2) Out breeding:
Out breeding is the breeding of the unrelated animals. Out breeding is of three types (a) Out-crossing (b) Cross-breeding (c) Interspecific hybridisation.
a) Out-crossing :
Mating of animals within the same breed, but having no common ancestors on either side of pedigree for 4-6 generations. The off spring of such mating is known as an out-cross.
b) Cross-breeding :
In this method, superior males of one breed are mated with superior females of another breed. The offspring of such a mating is said to be a cross breed.
c) Interspecific hybridisation :
In this method, male and female animals of two different related species are mated. The progeny may combine desirable features of both the parents and is different from both the parents.
Question 2.
Define the term breed. What are the objectives of animal breeding ?
Answr:
Breed:
A breed is a group of animals related by descent and similar in most characters such as general appearance, size, configuration and features with other members of the same species.
Jersery and Brown Swiss are example of foreign breeds of cattle. These two varieties of cattle have the ability to produce abundant quantities of milk. This milk is very nutritious with high protein content.
Objects of animal breeding :
- To produce disease resistant animals.
- Increase in the quality and quantity of milk, meat, wool etc.,
- Fast growth rate.
- Enhanced productive life by improving the genetic merit of livestock.
- Early maturity
- Economy of feed
Question 3.
Explain the role of animal husbandry in human welfare.
Answer:
Animal husbandary deals with the scientific management of livestock. It includes various aspects such as feeding, breeding and control diseases to raise the population of livestock. Animal husbandary usually includes buffaloes, cows, pigs, horses, cattle, sheep, camels, goats, poultry, fish etc which are useful for humans in various ways.
These animals are managed for production of commercially important products such as milk, meat, wool, egg, honey, silk etc. The increase in human population has increased the demand of these products. Hence it is necessary to improve the management of livestock scientifically. ,
Question 4.
List out the various steps involved in MOET.
Answer:
The following are the steps involved in Multiple Ovulation and Embryo Transfer /MOET):
- A cow is administrated hormones, with FSH like activity.
- This induces follicular maturation and super ovulation.
- In Super ovulation instead of one egg, which they produce per cycle, they produce 6 – 8 eggs.
- The cow is either mated with elite bull or artificially inseminated.
- The embryos are at 8-32 called stages are recovered non-aurgically and transferred to surrogate mother, when the embryo develops into complete animal.
Now the genetic mother is ready for another round of super ovulation. This technology is in use for cattle, sheep, rabbits, buffaloes etc. to produce high yielding ones.
Question 5.
Write short notes on controlled breeding experiments.
Answer:
Controlled breeding experiments are carried out using artificial insemination and multiple ovulation and embryo transfer technology.
- In this technique the semen is collected from superior bulls. This semen can be used immediately or can be frozen and used later period. It can be transported in a frozen form to place where a female is housed.
- Meanwhile a cow or animal is administered hormones, with FSH like activity.
- These hormones induces follicular maturation and super ovulation.
- Now the cow is artificially inseminated for fertilisation.
- The embryos are at 8-32 celled stages are recovered non-surgically and transferred to surrogate mother uterus for further development.
This technology is use for cattle, sheep, rabbits, buffaloes etc. By using this method we can produce high milk and meat yielding animals and also control the venereal diseases.
![]()
Question 6.
Explain the important components of poultry management.
Answer:
Important components of poultry management:
Selection of disease free and suitable breeds:
The selected’breeds should be disease free and get acclimatised to a wide range of climatic conditions. Eg: In India Hybrid layers-BV 300, Hyline, Poona – Pearls etc., Broiler strains – Hubbard, Vencobb etc.
Feed management:
Balanced diet is must to maximise the yield. Brooder, chick mash, grower mash, prelayer mash and layer mash are fed to layers at different stages. Likewise pre starter mash, starter mash and finish mash are the feed given to broilers. Safewater should be supplied through waterers at all times.
Health care :
Vaccination against viral diseases and using antibodies for both bacterial and fungal diseases.
In addition to the above hygiene, proper and safe farm conditions ensure better produce.
Question 7.
Discuss in brief about ‘AvianFlu’.
Answer:
AvianFlu or birdFlu is an important disease affecting poultry birds and man.
Causative organism :
AvianFlu or birdFlu is caused by an “avianFlu virus” the H5NI. The virus that causes the bird infection infects humans too. It is a pandemic disease.
Mode of infection:
Infection may be spread simply by touching contaminated surfaces. Birds infected by this type of influenza, continue to release the virus as in their faeces and saliva for as long as 10 days.
Symptoms:
In humans it causes typical-flu-like symptoms, include cough, diarrhoea difficulty in breathing, fever, headache, malaise, muscle aches and sore throat.
Prevention :
- Avoiding consumption of under cooked chicken.
- People who work for poultry birds should use protective clothing and special breathing masks.
- Complete culling of infected flock by burying or burning them.
Question 8.
Explain in brief about queen bee.
Answer:
- Queen bee is the largest individual in the colony.
- It is a fertile diploid female, one per bee hive and the egg layer of the colony.
- She lives for about five years and her only function is to lay eggs.
- The queen bee during its nuptial flight receives sperms from a drone and stores in the spermathecae and lays two types of eggs, the fertilised and unfertilised.
- All fertilised eggs develop into females.
- All the larvae developing from the fertilised eggs are fed with the royal jelly for first four days only. Afterwards royal jelly is fed only to the bee that is bound to develop into next queen, whereas the other larvae fed on bee bread become workers.
Question 9.
Honey bees are economically important – justify.
Answer:
- Honeybees are economically important insects in the world. Because honeybee products like Honey, wax, propolis and beevenom have more economic importance.
- Honey – It is a rich source of fructose, glucose, water minerals and vitamins.
- Bee’s wax – It is used in the preparation of cosmetics, polishes of various kinds and candles.
- Propolis – Propolis is used in the treatment of inflammation and superficial bums.
- Bee’s Venom – Which extracted .from the string of worker bees is used in the treatment of rheumatoid arthritis.
- Pollination – Bees are the pollinators of our crop plants such as sunflower, Brassica, Apple and Pear.
Question 10.
What are the various factors required for Bee keeping?
Answer:
Bee keeping or apiculture is the maintenance of hives of honeybees for the production of honey and wax.
Factors required for successful Bee keeping :
- Knowledge of nature and habits of honeybees.
- Selection of suitable location for keeping the beehives. ‘
- Raising a hive with the help of a queen and small group of worker bees.
- Management of beehives during different seasons.
- Knowledge of handling procedures and collection of honey and bee wax.
Question 11.
Fisheries have carved a niche in Indian economy. Explain.
Answer:
Fisheries have carved a niche in Indian economy, as fisheries have more economic importance.
As food :
Fish meat, in general is a good source of proteins, vitamins, minerals and rich in iodine. Tunas, shrimps and crabs are not only edible but also have export value.
Byproducts :
- Shark and Cod liver oils – are good source of vitamins A and D.
Oils from Sardine and Salmon- are good source of Omega 3 – fatty acids. - Fish guano from Scarp fish – used as fertilizer.
- Shagree and I$inglass – used in clarification of wines.
Question 12.
Explain in brief structure of Insulin.
Answer:
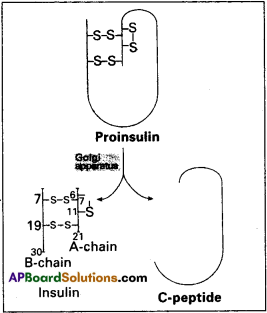
Insulin is a poly peptide hormone produced by the β – cells of islets of langerhans of pancreas. It is the first protein produced by recombinant DNA technology.
Structure of Insulin :
Human insulin is made up of 51 aminoacids arranged in two polypeptide chains. The chain A has 21 aminoacids while chain B has 30 aminoacids. Both are held together by two interchain disulfide bridges, connecting A7 to B7 arid A20 to B19. In addition, there is an intrachain disulfide link in chain A between the aminoacids 6 and 11.
In mammals, including humans, insulin is synthesized as a pro-hormone, which contains an extra stretch called the ‘c’ peptide. This ‘c’ peptide is not present in the mature insulin and is removed during maturation into insulin. .
Question 13.
Define Vaccine and discuss about types of Vaccines.
Answer:
A Vaccine is a biological preparation that improves immunity to a particular diseases. A Vaccine typically contains inactive or attenuated disease causing microorganisms. The toxin or one of the surface proteins of the microorganisms are also used in preparing vaccines.
Types of Vaccines :
1) Attenuated whole agent vaccines :
They contain disabled line microorganisms. Mostly they are antiviral. Eg: Vaccines against Yellow fever, measles, rubella and mumps and the bacterial disease such as typhoid.
2) Inactivated whole agent vaccines :
They contain killed microbes. Eg : Vaccines against influenza, cholera, hepatitis A, rabies etc.
3) Toxoids:
They contain toxoids which are inactivated exotoxins of certain microbes.
Eg : The vaccines against diphtheria and tetanus.
Question 14.
Write in brief the types of gene therapy. .
Answer:
Gene therapy is the insertion of genes into an individual’s cells and tissues to treat a
There are two approaches to achieve gene therapy :
- Somatic line therapy
- Germ line gene therapy
1) Somatic line therapy:
In this type of therapy, functional genes are introduced into somatic cells of a patient. The approach is to correct a disease phenotype by treating defect in somatic cells in the affected person. The changes effected in this type of gene therapy are nonfinheritable.
Somatic line therapy is of two types :
a) Ex-vivo gene therapy:
In which the cell are collected from patient, modified outside the body and then transplanted back Eg: SCID.
b) In-vivo gene therapy :
In this therapy, the genes are changed in cells, while they are still inside the body Eg : Cystic fibrosis.’
2) Germ line gene therapy:
In this type of therapy, functional genes are introduced into sperms or ova and are thus integrated into their genomes. Therefore the changes or modifications become heritable. Due to various technical and ethical reasons, the germ line gene therapy remained at infant stage.
Question 15.
List out any four salient features of cancer cells.
Answer:
Salient features of Cancer cells :
Loss of contact inhibition :
Normal cells in a culture stop growing when their plasma membranes come into contact with one another. This inhibition of growth after contact is called contact inhibition. Cancer cells lose this property.
Reduced intracellular adhesion :
When normal cells growing in medium, the cells are joined by intracellular adhesion proteins called cadherins. They are missing in Cancer cells.
Immortalisation :
Normal cell culture does not survive indefinitely. They undergo apoptosis. Where as Cancer cells do not undergo apoptosis.
Loss of anchorage dependence :
Most normal cells must be attached to a rigid substratum in order to grow Cancer cells can grow even when they are not attached to the substratum.
Increased growth of blood vessels :
When tumors grow in size diffusion of oxygen and nutrients become restricted and so tumors resort to attracting more blood vessels from their surrounding matrix.
![]()
Question 16.
Explain the different types of cancers.
Answer:
Based on the origin Cancers are classified into :
1) Carcinomas :
These are malignant tumor of epithelial cells. They are originating from the epithelial tissues of skin, lining of the respiratory, digestive, urinary and genejal systems or cells from various glands breast and nervous tissue etc. 85% of Cancers are Carcinomas.
2) Sarcomas :
These are malignant tumors of connective tissues or organs that originate from mesoderm. About 2% of tumors are Sarcomas.
3) Leukemias :
These are malignant tumors of stem ceils of hematopoietic tissues, resulting in unrestrained production of WBC. They are liquid tumors. About 4% of Cancers are Leukemias.
4) Lymphomas :
These are malignant tumors of secondary lymphoid organs like spleen, and lymphnodes. About 4% of Cancers are Lymphomas.
Question 17.
Write about the procedure involved in MRI. X jmsin
Answer:
MRI Scan is a diagnostic radiology technique that uses magnetism, radiowaves and a computer to produce images of body components.
Procedure :
MRI Scanner is giant circular magnetic tube.
- The patient is placed on a movable bed that is inserted into the magnet.
- Human body is mainly composed of water which contains two protons.
- The magnet creates a strong magnetic field that makes these proton align with the direction of the magnetic field.
- A second radiofrequency electromagnetic field is then turned on for a brief period. The protons absorb some energy from these radio waves.
- When this second radio frequency emitting field is turned off, the protons release energy at a radio frequency which can be detected by the MRI scanner.
- Different types of tissues emit different quanta of energy. Abnormal tissues such as tumors can be detected because the protons in different types of tissues return to their equilibrium state at different rates.
- Tissues of bone with less water content look different in MRI, and pathological tissues also can be detected.
The information received is processed by computer and generated an image.
Question 18.
Write briefly about different waves and intervals in an ECG. X
Answer:
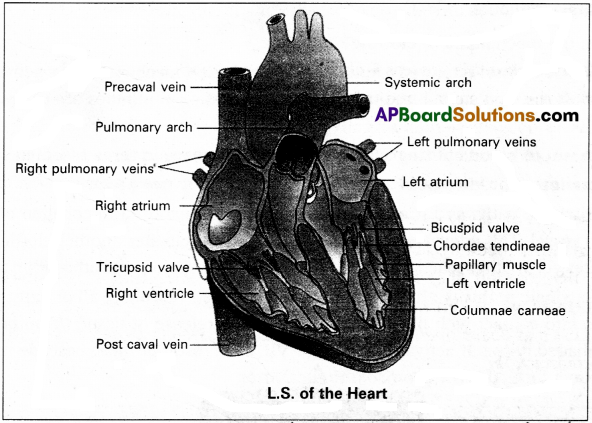
ECG (electrocardiography) is commonly used, non-invasive procedure fro recording electrical changes in the heart.
The graphic record which is called an electrocardiogram, shows the series of waves that occur during each cardiac cycle.
The normal ECG consists of (i) Waves (ii) Intervals (iii) Segments (iv) Complexes.
i) Waves :
- The waves in a normal record are named P, Q, R, S and T in that order.
- A typical ECG tracing of a normal heartbeat consists of (I) a ’P’ wove (II) a ‘QRS complex of waves’ (III) a T Wave.
- P wave: It represents the atrial systole and shows that the impulse is passing through atria. The duration of P. Wave is 0.1 sec.
- QRS complex of wave : It represents ventricular systole. Q wave is small negative., R-wave is tall positive and S wave is a negative wave. Its duration is 0.08 to 0.1 sec.
- T wave: It represents the ventricular repolarization. It is a positive wave,’its duration is 0.2 sec.
ii) Intervals:
P-R intervals :
P-R intervals is the interval between the onset of p wave and the onset of Q wave. P-R interval is normally. 0.12 – 02 sec.
Q-T intervals :
The interval between the onset of Q wave and the end of the T-wave. It represents the electrical activity in muscle of the ventricles. It lasts for about 0.4 seconds.
R-R intervals:
It signifies the duration of one cardiac cycle and lasts for about 0.8 sec
Segments :
S-T segment is the time period between the end of the ‘S’ wave and the onset of the T-wave. It is an isoelectric or zero voltage period.
Question 19.
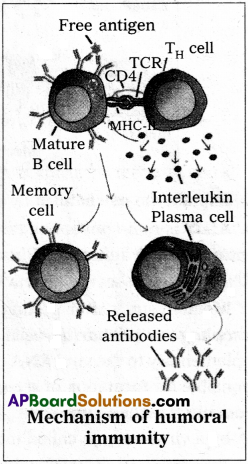
Discuss briefly the process of indirect ELISA.
Answer:
Enzyme linked immunosorbent assay is a tool of clinical immunology to detect, antigens or antibodies in a given sample. ELISA is of two types (1) Direct ELISA (2) Indirect EUSA.
Indirect ELISA:
It is used to detect antibodies present in the serum of the patient or given sample.
Protocol
- A known antigen is added to the well, which absorbed on the surface of well.
- Patients antiserum is added to AG coated well.
- Allowed to react antibodies present in the serum with the antigen, coated on the surface of the well.
- Washed the well to remove the any unbound free antibodies present in the well.
- Enzyme linked antihuman serum globulins are added. They bind to the antibody which is already bound to the antigen.
- Washed it to remove excess antibodies present m the well.
- Enzyme substrate is added and the reaction produces a visible colour change which can be measured by a spectro photometer.
If there are no antibodies (i.e., anti HIV antibodyies in the serum sample, there is no binding of primary antibodies to the antigens and so enzyme linked secondary antibodies do not bind to the primary antibodies. There cannot be any enzymatic reaction and so no colour change is observed the test is said to be negative.
Question 20.
Write short note on EEG.
Answer:
Electro encephalography is the process of recording the electrical activity of the brain with help of an EEG machine and some electrodes placed all over the scalp.
The waves recorded by an EEG consist of synchronized waves which are common in normal healthy people and, in certain neurological conditions the waves are desynchronized. The wave pattern can be broadly classified into alpha, beta, delta and theta wave pattern.
Alpha waves :
They are rhythmical 8-13 cycleslsec. This type of Wave pattern is seen in persons who are drowsy or sleepy with closed eyes.
Beta waves:
These waves occur at a high frequency of 13-40 cycleslsec their amplitude is low. There are desynchronized waves recorded in person who are mentally very active and tense.
Delta waves :
Their frequency is quite low i.e., less than 3 cycleslsec. They have high amplitude. They are common in early childhood in awaken condition. In adults, they occur in deep sleep, epilepsy, mental depression etc. .
Theta waves:
Their frequency is between 4 and 7 cycleslsec. These waves are common in children of less than 5 years of age and emotional stress in adults.
Uses :
- EEG is useful tool in diagnosing neurological apd sleep disorders.
- The diagnostic application of EEG is the diagnosis of epilepsy.
- EEG is also useful in the diagnosis of coma and brain death.
Long Answer Questions
Question 1.
Write in detail about outbreeding.
Answer:
Out breeding is the breeding of the unrelated animals, it is the cross between different breeds.
Out breeding is of three types
- Out crossing
- Cross breeding
- Inter specific hybridisation.
1) Out crossing :
It is the practice of mating of animals with in the same breed, but having no common ancestors on either side of the pedigree for 4-6 generations. The offspring of such a mating is known as an outcroas. It is the best breading method for animals that are below average in milk production, growth rate etc.
2) Cross breeding :
In this method, superior males of one breed are mated with superior females of another breed. The offspring of such a mating is said to be a cross breed. Cross breeding allows the desirable qualities of two different breeds to be combined. The progeny is not only used for commercial production but also inbreeding and selection to develop stable breeds which may be superior to existing breeds.
Eg : Hisardale is a new breed of sheep developed by crossing Bikaneri ewes and Marino rams. ‘ . .
3) Inter specific hybridisation :
In this method, male and female animals of two different related species are mated. The progeny may combine desirable features of both the parents and is different from both the parents.
Eg: 1) When a male donkey is crossed with a female horse, it leads to the production of “mule” (sterile/
2) When a male horse is crossed with a female donkey “hinny” (sterile) is produced. Mules have considerable economic value.
![]()
Question 2.
Explain in detail clinical inferences from ECG. –
Answer:
ECG is commonly used, non-invassive procedure for recording electrical changes in the heart The graphic record is called an electrocardiogram, shows the series of waves that occur during each cardiac cycle.
Normal ECG consist of waves, intervals, segments and complexes.
Waves :
A typical ECG tracing normal heart beat consist of a ‘P’ wave a QRS complex of waves, a T wave.
P wave :
It represents the atrial systole and shows that the impulse is passing through atria. The duration of P wave is 0./ sec.
QRS complex of wave:
It represents ventricular systole Q wave is small negative, R-Wave is tall positive and S-yvave is a negative wave. It’s donation is 0.08 to 0.1 sec.
T wave :
It represents the ventricular repolarization. It is a positive wave, its duration is 0.2 sec.
Intervals:
P – R intervals :
It is the interval between 9nset of P wave and onset of Q wave. P-R interval is normally 0.12-0.2 sec.
Q – T intervals :
The interval between the onset of Q wave and the end of the • T-wave. It represents the electrical activity in muscle of the ventricles. It lasts for about 0.4 sec.
R – R intervals :
It signifies the duration of one cardiac cycle and lasts for about 0.8 sec . .
Segments :
S-T segment is the time-period between the end of the ‘S’ wave and the onset of the T-wave. it is an isoelectric or zero voltage period.
Clinical inferences from ECG :
- Enlarged P wave – indicates enlarged atria
- Variation in the duration, amplitude and morphology of the QRS complex – indicates disorders such as block of conduction of impulses through the branches of the bundle of His.
- Prolonged P-R interval duration – indicates delay in conduction of impulses from S-A node to the A – V node.
P-R interval is prolonged in bradycardia and shortened in tachycardia. - Prolonged Q-T interval – indicates myocardial infraction and hypothyroidism.
- Shortened Q.T interval – indicates hyper calcemio.
- Elevated S – T segment – indicates myocardial infarction.
- Tall T wave – indicates hyperkalemia.
- Small, flat or inverted T wave – indicates hypokalemia.